G Protein-Coupled Receptor Signaling in Stem Cells and Cancer
- PMID: 27187360
- PMCID: PMC4881529
- DOI: 10.3390/ijms17050707
G Protein-Coupled Receptor Signaling in Stem Cells and Cancer
Abstract
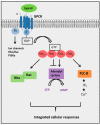
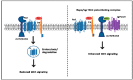
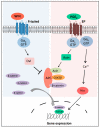
G protein-coupled receptors (GPCRs) are a large superfamily of cell-surface signaling proteins that bind extracellular ligands and transduce signals into cells via heterotrimeric G proteins. GPCRs are highly tractable drug targets. Aberrant expression of GPCRs and G proteins has been observed in various cancers and their importance in cancer stem cells has begun to be appreciated. We have recently reported essential roles for G protein-coupled receptor 84 (GPR84) and G protein subunit Gαq in the maintenance of cancer stem cells in acute myeloid leukemia. This review will discuss how GPCRs and G proteins regulate stem cells with a focus on cancer stem cells, as well as their implications for the development of novel targeted cancer therapies.
Keywords: G proteins; GPCR; cancer stem cells; signaling; stem cells; therapy.
Figures

References
-
- Reubinoff B.E., Pera M.F., Fong C.Y., Trounson A., Bongso A. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat. Biotechnol. 2000;18:399–404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

