Effect of AQP9 Expression in Androgen-Independent Prostate Cancer Cell PC3
- PMID: 27187384
- PMCID: PMC4881560
- DOI: 10.3390/ijms17050738
Effect of AQP9 Expression in Androgen-Independent Prostate Cancer Cell PC3
Abstract
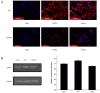
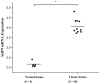
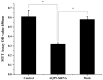
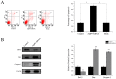
It is known that aquaporin 9 (AQP9) in the prostate was strictly upregulated by androgen and may represent a novel therapeutic target for several cancers, but whether AQP9 plays a role in the regulation of androgen-independent prostate cancer still remains unclear. In the present study, AQP9 was determined in prostate cancer and adjacent cancer tissues; AQP9-siRNA was applied to silencing AQP9 in androgen-independent prostate cancer cell PC3 cell line. Western blot and flow cytometry analysis were employed to detect changes in related-function of control and AQP9-siRNA groups. The results showed that AQP9 is significantly induced in cancer tissues than that in adjacent cancer tissues. Moreover, knockdown of AQP9 in PC3 androgen-independent prostate cancer cell prostate cancer cells increased inhibition rates of proliferation. In addition, knockdown of AQP9 resulted in a significant decrease in the expression of the Bcl-2 and with a notable increase in the expression of Bax and cleaved caspase 3, indicated that AQP9 knockdown promoted apoptosis in prostate cancer cells. From wound healing assay and matrigel invasion, we suggested that AQP9 expression affects the motility and invasiveness of prostate cancer cells. Moreover, In order to explore the pathway may be involved in AQP9-mediated motility and invasion of prostate cancer cells, the phosphorylation of ERK1/2 was significant suppressed in AQP9 siRNA-transfected cells compared with that in control cells, suggesting that AQP9 is involved in the activation of the ERK pathway in androgen-independent prostate cancer cells.
Keywords: ERK signaling pathway; RNA interference; apoptosis; aquaporin 9; invasion; proliferation; prostate cancer.
Figures



Similar articles
-
Wnt-11 promotes neuroendocrine-like differentiation, survival and migration of prostate cancer cells.Mol Cancer. 2010 Mar 10;9:55. doi: 10.1186/1476-4598-9-55. Mol Cancer. 2010. PMID: 20219091 Free PMC article.
-
Androgen receptor as a regulator of ZEB2 expression and its implications in epithelial-to-mesenchymal transition in prostate cancer.Endocr Relat Cancer. 2014 May 8;21(3):473-86. doi: 10.1530/ERC-13-0514. Print 2014 Jun. Endocr Relat Cancer. 2014. PMID: 24812058
-
Overexpression of HepaCAM inhibits cell viability and motility through suppressing nucleus translocation of androgen receptor and ERK signaling in prostate cancer.Prostate. 2014 Jul;74(10):1023-33. doi: 10.1002/pros.22817. Epub 2014 May 8. Prostate. 2014. PMID: 24811146
-
MHY-449, a novel dihydrobenzofuro[4,5-b][1,8] naphthyridin-6-one derivative, induces apoptotic cell death through modulation of Akt/FoxO1 and ERK signaling in PC3 human prostate cancer cells.Int J Oncol. 2014 Mar;44(3):905-11. doi: 10.3892/ijo.2014.2257. Epub 2014 Jan 10. Int J Oncol. 2014. PMID: 24424889
-
Apoptosis, tumour invasion and prostate cancer.Br J Urol. 1997 Apr;79 Suppl 2:27-34. doi: 10.1111/j.1464-410x.1997.tb16918.x. Br J Urol. 1997. PMID: 9126067 Review. No abstract available.
Cited by
-
Regulation mechanism of aquaporin 9 gene on inflammatory response and cardiac function in rats with myocardial infarction through extracellular signal-regulated kinase1/2 pathway.Heart Vessels. 2019 Dec;34(12):2041-2051. doi: 10.1007/s00380-019-01452-8. Epub 2019 Jun 19. Heart Vessels. 2019. PMID: 31218464
-
AQP9 promotes astrocytoma cell invasion and motility via the AKT pathway.Oncol Lett. 2018 Nov;16(5):6059-6064. doi: 10.3892/ol.2018.9361. Epub 2018 Aug 23. Oncol Lett. 2018. PMID: 30344749 Free PMC article.
-
Mechanisms of Aquaporin-Facilitated Cancer Invasion and Metastasis.Front Chem. 2018 Apr 25;6:135. doi: 10.3389/fchem.2018.00135. eCollection 2018. Front Chem. 2018. PMID: 29922644 Free PMC article. Review.
-
The Association of Aquaporins with MAPK Signaling Pathway Unveils Potential Prognostic Biomarkers for Pancreatic Cancer: A Transcriptomics Approach.Biomolecules. 2025 Mar 26;15(4):488. doi: 10.3390/biom15040488. Biomolecules. 2025. PMID: 40305202 Free PMC article.
-
Patterns of Gene Expression Profiles Associated with Colorectal Cancer in Colorectal Mucosa by Using Machine Learning Methods.Comb Chem High Throughput Screen. 2024;27(19):2921-2934. doi: 10.2174/0113862073266300231026103844. Comb Chem High Throughput Screen. 2024. PMID: 37957897
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

