Respiratory Syncytial Virus and Cellular Stress Responses: Impact on Replication and Physiopathology
- PMID: 27187445
- PMCID: PMC4885079
- DOI: 10.3390/v8050124
Respiratory Syncytial Virus and Cellular Stress Responses: Impact on Replication and Physiopathology
Abstract
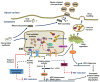
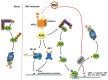
Human respiratory syncytial virus (RSV), a member of the Paramyxoviridae family, is a major cause of severe acute lower respiratory tract infection in infants, elderly and immunocompromised adults. Despite decades of research, a complete integrated picture of RSV-host interaction is still missing. Several cellular responses to stress are involved in the host-response to many virus infections. The endoplasmic reticulum stress induced by altered endoplasmic reticulum (ER) function leads to activation of the unfolded-protein response (UPR) to restore homeostasis. Formation of cytoplasmic stress granules containing translationally stalled mRNAs is a means to control protein translation. Production of reactive oxygen species is balanced by an antioxidant response to prevent oxidative stress and the resulting damages. In recent years, ongoing research has started to unveil specific regulatory interactions of RSV with these host cellular stress responses. Here, we discuss the latest findings regarding the mechanisms evolved by RSV to induce, subvert or manipulate the ER stress, the stress granule and oxidative stress responses. We summarize the evidence linking these stress responses with the regulation of RSV replication and the associated pathogenesis.
Keywords: ER stress; RSV; endoplasmic reticulum; inclusion bodies; oxidative stress; reactive oxygen species; respiratory syncytial virus; stress granule; stress response; virus.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

