Two Novel Dermaseptin-Like Antimicrobial Peptides with Anticancer Activities from the Skin Secretion of Pachymedusa dacnicolor
- PMID: 27187467
- PMCID: PMC4885059
- DOI: 10.3390/toxins8050144
Two Novel Dermaseptin-Like Antimicrobial Peptides with Anticancer Activities from the Skin Secretion of Pachymedusa dacnicolor
Abstract
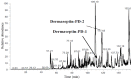
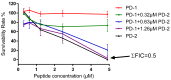
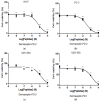
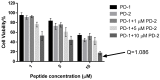
The dermaseptin antimicrobial peptide family contains members of 27-34 amino acids in length that have been predominantly isolated from the skins/skin secretions of phyllomedusine leaf frogs. By use of a degenerate primer in Rapid amplification of cDNA ends (RACE) PCR designed to a common conserved domain within the 5'-untranslated regions of previously-characterized dermaseptin encoding cDNAs, two novel members of this peptide family, named dermaseptin-PD-1 and dermaseptin-PD-2, were identified in the skin secretion of the phyllomedusine frog, Pachymedusa dacnicolor. The primary structures of both peptides were predicted from cloned cDNAs, as well as being confirmed by mass spectral analysis of crude skin secretion fractions resulted from reversed-phase high-performance liquid chromatography. Chemically-synthesized replicates of dermaseptin-PD-1 and dermaseptin-PD-2 were investigated for antimicrobial activity using standard model microorganisms (Gram-positive bacteria, Gram-negative bacteria and a yeast) and for cytotoxicity using mammalian red blood cells. The possibility of synergistic effects between the two peptides and their anti-cancer cell proliferation activities were assessed. The peptides exhibited moderate to high inhibition against the growth of the tested microorganisms and cancer cell lines with low haemolytic activity. Synergistic interaction between the two peptides in inhibiting the proliferation of Escherichia coli and human neuronal glioblastoma cell line, U251MG was also manifested.
Keywords: amphibian; anticancer; antimicrobial; molecular cloning; peptide.
Figures

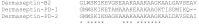



Similar articles
-
Dermaseptin-PH: A Novel Peptide with Antimicrobial and Anticancer Activities from the Skin Secretion of the South American Orange-Legged Leaf Frog, Pithecopus (Phyllomedusa) hypochondrialis.Molecules. 2017 Oct 24;22(10):1805. doi: 10.3390/molecules22101805. Molecules. 2017. PMID: 29064402 Free PMC article.
-
Evaluating the Bioactivity of a Novel Antimicrobial and Anticancer Peptide, Dermaseptin-PS4(Der-PS4), from the Skin Secretion of Phyllomedusa sauvagii.Molecules. 2019 Aug 16;24(16):2974. doi: 10.3390/molecules24162974. Molecules. 2019. PMID: 31426323 Free PMC article.
-
A Novel Dermaseptin Isolated from the Skin Secretion of Phyllomedusa tarsius and Its Cationicity-Enhanced Analogue Exhibiting Effective Antimicrobial and Anti-Proliferative Activities.Biomolecules. 2019 Oct 18;9(10):628. doi: 10.3390/biom9100628. Biomolecules. 2019. PMID: 31635388 Free PMC article.
-
The dermaseptin superfamily: a gene-based combinatorial library of antimicrobial peptides.Biochim Biophys Acta. 2009 Aug;1788(8):1537-50. doi: 10.1016/j.bbamem.2008.09.006. Epub 2008 Sep 26. Biochim Biophys Acta. 2009. PMID: 18929530 Review.
-
Dermaseptins as models for the elucidation of membrane-acting helical amphipathic antimicrobial peptides.Curr Pharm Biotechnol. 2011 Aug;12(8):1184-93. doi: 10.2174/138920111796117319. Curr Pharm Biotechnol. 2011. PMID: 21470155 Review.
Cited by
-
Peptide-Protein Interaction Studies of Antimicrobial Peptides Targeting Middle East Respiratory Syndrome Coronavirus Spike Protein: An In Silico Approach.Adv Bioinformatics. 2019 Jul 1;2019:6815105. doi: 10.1155/2019/6815105. eCollection 2019. Adv Bioinformatics. 2019. PMID: 31354813 Free PMC article.
-
Peptidoglycan potentiates the membrane disrupting effect of the carboxyamidated form of DMS-DA6, a Gram-positive selective antimicrobial peptide isolated from Pachymedusa dacnicolor skin.PLoS One. 2018 Oct 16;13(10):e0205727. doi: 10.1371/journal.pone.0205727. eCollection 2018. PLoS One. 2018. PMID: 30325956 Free PMC article.
-
Recent Advances in Multifunctional Antimicrobial Peptides as Immunomodulatory and Anticancer Therapy: Chromogranin A-Derived Peptides and Dermaseptins as Endogenous versus Exogenous Actors.Pharmaceutics. 2022 Sep 22;14(10):2014. doi: 10.3390/pharmaceutics14102014. Pharmaceutics. 2022. PMID: 36297449 Free PMC article. Review.
-
Bioinspired Bola-Type Peptide Dendrimers Inhibit Proliferation and Invasiveness of Glioblastoma Cells in a Manner Dependent on Their Structure and Amphipathic Properties.Pharmaceutics. 2020 Nov 18;12(11):1106. doi: 10.3390/pharmaceutics12111106. Pharmaceutics. 2020. PMID: 33217976 Free PMC article.
-
Unlocking the potential of tumor-targeting peptides in precision oncology.Oncol Res. 2025 Jun 26;33(7):1547-1570. doi: 10.32604/or.2025.062197. eCollection 2025. Oncol Res. 2025. PMID: 40612874 Free PMC article. Review.
References
-
- Vanhoye D., Bruston F., Nicolas P., Amiche M. Antimicrobial peptides from hylid and ranin frogs originated from a 150-million-year-old ancestral precursor with a conserved signal peptide but a hypermutable antimicrobial domain. Eur. J. Biochem. 2003;270:2068–2081. doi: 10.1046/j.1432-1033.2003.03584.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

