The Diversity of Ribonuclease P: Protein and RNA Catalysts with Analogous Biological Functions
- PMID: 27187488
- PMCID: PMC4919922
- DOI: 10.3390/biom6020027
The Diversity of Ribonuclease P: Protein and RNA Catalysts with Analogous Biological Functions
Abstract
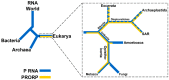
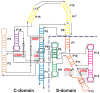
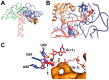
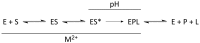
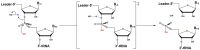
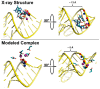
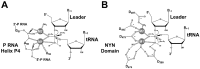
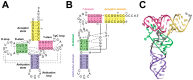
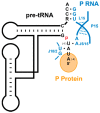
Ribonuclease P (RNase P) is an essential endonuclease responsible for catalyzing 5' end maturation in precursor transfer RNAs. Since its discovery in the 1970s, RNase P enzymes have been identified and studied throughout the three domains of life. Interestingly, RNase P is either RNA-based, with a catalytic RNA subunit, or a protein-only (PRORP) enzyme with differential evolutionary distribution. The available structural data, including the active site data, provides insight into catalysis and substrate recognition. The hydrolytic and kinetic mechanisms of the two forms of RNase P enzymes are similar, yet features unique to the RNA-based and PRORP enzymes are consistent with different evolutionary origins. The various RNase P enzymes, in addition to their primary role in tRNA 5' maturation, catalyze cleavage of a variety of alternative substrates, indicating a diversification of RNase P function in vivo. The review concludes with a discussion of recent advances and interesting research directions in the field.
Keywords: PRORP; RNase P; endonuclease; ribozyme; tRNA maturation; tRNA recognition.
Figures
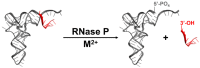

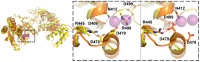
Similar articles
-
Mechanistic Studies Reveal Similar Catalytic Strategies for Phosphodiester Bond Hydrolysis by Protein-only and RNA-dependent Ribonuclease P.J Biol Chem. 2015 May 22;290(21):13454-64. doi: 10.1074/jbc.M115.644831. Epub 2015 Mar 27. J Biol Chem. 2015. PMID: 25817998 Free PMC article.
-
Structure of ribonuclease P--a universal ribozyme.Curr Opin Struct Biol. 2006 Jun;16(3):327-35. doi: 10.1016/j.sbi.2006.04.002. Epub 2006 May 2. Curr Opin Struct Biol. 2006. PMID: 16650980 Review.
-
RNase P enzymes: divergent scaffolds for a conserved biological reaction.RNA Biol. 2013 Jun;10(6):909-14. doi: 10.4161/rna.24513. Epub 2013 Apr 1. RNA Biol. 2013. PMID: 23595059 Free PMC article.
-
Molecular recognition of pre-tRNA by Arabidopsis protein-only Ribonuclease P.RNA. 2017 Dec;23(12):1860-1873. doi: 10.1261/rna.061457.117. Epub 2017 Sep 5. RNA. 2017. PMID: 28874505 Free PMC article.
-
Structural and mechanistic basis of RNA processing by protein-only ribonuclease P enzymes.Trends Biochem Sci. 2022 Nov;47(11):965-977. doi: 10.1016/j.tibs.2022.05.006. Epub 2022 Jun 18. Trends Biochem Sci. 2022. PMID: 35725940 Review.
Cited by
-
Catalytic RNA Oligomers Formed by Co-Oligomerization of a Pair of Bimolecular RNase P Ribozymes.Molecules. 2022 Nov 28;27(23):8298. doi: 10.3390/molecules27238298. Molecules. 2022. PMID: 36500390 Free PMC article.
-
RNA binding protein POP7 regulates ILF3 mRNA stability and expression to promote breast cancer progression.Cancer Sci. 2022 Nov;113(11):3801-3813. doi: 10.1111/cas.15430. Epub 2022 Sep 6. Cancer Sci. 2022. PMID: 35579257 Free PMC article.
-
Grad-seq analysis of Enterococcus faecalis and Enterococcus faecium provides a global view of RNA and protein complexes in these two opportunistic pathogens.Microlife. 2022 Dec 27;4:uqac027. doi: 10.1093/femsml/uqac027. eCollection 2023. Microlife. 2022. PMID: 37223738 Free PMC article.
-
Distributive enzyme binding controlled by local RNA context results in 3' to 5' directional processing of dicistronic tRNA precursors by Escherichia coli ribonuclease P.Nucleic Acids Res. 2019 Feb 20;47(3):1451-1467. doi: 10.1093/nar/gky1162. Nucleic Acids Res. 2019. PMID: 30496557 Free PMC article.
-
Repairing tRNA termini: News from the 3' end.RNA Biol. 2016 Dec;13(12):1182-1188. doi: 10.1080/15476286.2016.1239007. Epub 2016 Sep 23. RNA Biol. 2016. PMID: 27661287 Free PMC article.
References
-
- The Nobel Prize in Chemistry 1989. [(accessed on 15 January 2016)]. Available online: http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1989/
-
- Robertson H.D., Altman S., Smith J.D. Purification and properties of a specific Escherichia coli ribonuclease which cleaves a tyrosine transfer ribonucleic acid precursor. J. Biol. Chem. 1972;247:5243–5251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

