Powerful Complex Immunoadjuvant Based on Synergistic Effect of Combined TLR4 and NOD2 Activation Significantly Enhances Magnitude of Humoral and Cellular Adaptive Immune Responses
- PMID: 27187797
- PMCID: PMC4871337
- DOI: 10.1371/journal.pone.0155650
Powerful Complex Immunoadjuvant Based on Synergistic Effect of Combined TLR4 and NOD2 Activation Significantly Enhances Magnitude of Humoral and Cellular Adaptive Immune Responses
Abstract
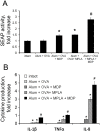
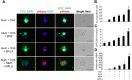
Binding of pattern recognition receptors (PRRs) by pathogen-associated molecular patterns (PAMPs) activates innate immune responses and contributes to development of adaptive immunity. Simultaneous stimulation of different types of PRRs can have synergistic immunostimulatory effects resulting in enhanced production of molecules that mediate innate immunity such as inflammatory cytokines, antimicrobial peptides, etc. Here, we evaluated the impact of combined stimulation of PRRs from different families on adaptive immunity by generating alum-based vaccine formulations with ovalbumin as a model antigen and the Toll-like receptor 4 (TLR4) agonist MPLA and the Nucleotide-binding oligomerization domain-containing protein 2 (NOD2) agonist MDP adsorbed individually or together on the alum-ovalbumin particles. Multiple in vitro and in vivo readouts of immune system activation all showed that while individual PRR agonists increased the immunogenicity of vaccines compared to alum alone, the combination of both PRR agonists was significantly more effective. Combined stimulation of TLR4 and NOD2 results in a stronger and broader transcriptional response in THP-1 cells compared to individual PRR stimulation. Immunostimulatory composition containing both PRR agonists (MPLA and MDP) in the context of the alum-based ovalbumin vaccine also enhanced uptake of vaccine particles by bone marrow derived dendritic cells (BMDCs) and promoted maturation (up-regulation of expression of CD80, CD86, MHCII) and activation (production of cytokines) of BMDCs. Finally, immunization of mice with vaccine particles containing both PRR agonists resulted in enhanced cellular immunity as indicated by increased proliferation and activation (IFN-γ production) of splenic CD4+ and CD8+ T cells following in vitro restimulation with ovalbumin and enhanced humoral immunity as indicated by higher titers of ovalbumin-specific IgG antibodies. These results indicate that combined stimulation of TLR4 and NOD2 receptors dramatically enhances activation of both the humoral and cellular branches of adaptive immunity and suggests that inclusion of agonists of these receptors in standard alum-based adjuvants could be used to improve the effectiveness of vaccination.
Conflict of interest statement
Figures




References
-
- Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol. 1999. December 15;163(12):6448–54. - PubMed
-
- Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

