Ferric ions accumulate in the walls of metabolically inactivating Saccharomyces cerevisiae cells and are reductively mobilized during reactivation
- PMID: 27188213
- PMCID: PMC4945443
- DOI: 10.1039/c6mt00070c
Ferric ions accumulate in the walls of metabolically inactivating Saccharomyces cerevisiae cells and are reductively mobilized during reactivation
Abstract
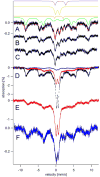
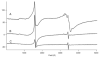
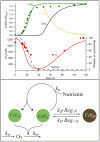
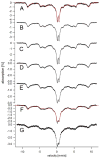
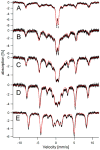
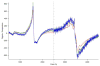
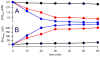
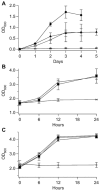
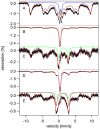
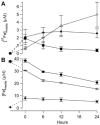
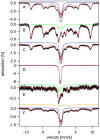
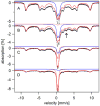
Mössbauer and EPR spectra of fermenting yeast cells before and after cell wall (CW) digestion revealed that CWs accumulated iron as cells transitioned from exponential to post-exponential growth. Most CW iron was mononuclear nonheme high-spin (NHHS) Fe(III), some was diamagnetic and some was superparamagnetic. A significant portion of CW Fe was removable by EDTA. Simulations using an ordinary-differential-equations-based model suggested that cells accumulate Fe as they become metabolically inactive. When dormant Fe-loaded cells were metabolically reactivated in Fe-deficient bathophenanthroline disulfonate (BPS)-treated medium, they grew using Fe that had been mobilized from their CWs AND using trace amounts of Fe in the Fe-deficient medium. When grown in Fe-deficient medium, Fe-starved cells contained the lowest cellular Fe concentrations reported for a eukaryotic cell. During metabolic reactivation of Fe-loaded dormant cells, Fe(III) ions in the CWs of these cells were mobilized by reduction to Fe(II), followed by release from the CW and reimport into the cell. BPS short-circuited this process by chelating mobilized and released Fe(II) ions before reimport; the resulting Fe(II)(BPS)3 complex adsorbed on the cell surface. NHHS Fe(II) ions appeared transiently during mobilization, suggesting that these ions were intermediates in this process. In the presence of chelators and at high pH, metabolically inactive cells leached CW Fe; this phenomenon probably differs from metabolic mobilization. The iron regulon, as reported by Fet3p levels, was not expressed during post-exponential conditions; Fet3p was maximally expressed in exponentially growing cells. Decreased expression of the iron regulon and metabolic decline combine to promote CW Fe accumulation.
Figures

References
-
- De Nobel JG, Klis FM, Priem J, Munnik T, VanDenEnde H. The gluconase-soluble mannoproteins limit cell wall porosity in Saccharomyces cerevisiae. Yeast. 1990;6:491–499. - PubMed
-
- DeGroot PWJ, Ram AF, Klis FM. Features and functions of covalently linked proteins in fungal cell walls. Fungal Genet Biol. 2005;42:657–675. - PubMed
-
- Klis FM, de Jong M, Brul S, de Groot PWJ. Extraction of cell surface-associated proteins from living yeast cells. Yeast. 2007;24:253–258. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

