Imaging follow-up after liver transplantation
- PMID: 27188846
- PMCID: PMC5124875
- DOI: 10.1259/bjr.20151025
Imaging follow-up after liver transplantation
Abstract
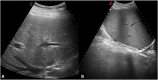
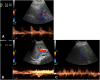
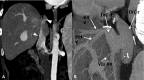
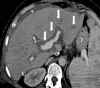
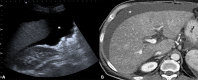
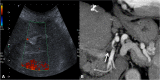
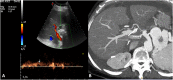
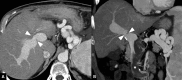
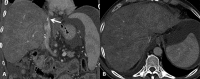
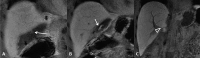
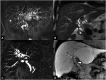
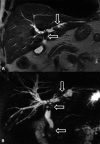
Liver transplantation (LT) represents the best treatment for end-stage chronic liver disease, acute liver failure and early stages of hepatocellular carcinoma. Radiologists should be aware of surgical techniques to distinguish a normal appearance from pathological findings. Imaging modalities, such as ultrasound, CT and MR, provide for rapid and reliable detection of vascular and biliary complications after LT. The role of imaging in the evaluation of rejection and primary graft dysfunction is less defined. This article illustrates the main surgical anastomoses during LT, the normal appearance and complications of the liver parenchyma and vascular and biliary structures.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources

