The effect of a decision aid on informed decision-making in the era of non-invasive prenatal testing: a randomised controlled trial
- PMID: 27189020
- PMCID: PMC5027684
- DOI: 10.1038/ejhg.2016.39
The effect of a decision aid on informed decision-making in the era of non-invasive prenatal testing: a randomised controlled trial
Abstract
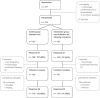
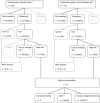
Early in pregnancy women and their partners face the complex decision on whether or not to participate in prenatal testing for fetal chromosomal abnormalities. Several studies show that the majority of pregnant women currently do not make informed decisions regarding prenatal testing. As the range of prenatal tests is expanding due to the development of new techniques such as non-invasive prenatal testing (NIPT), autonomous reproductive decision-making is increasingly challenging. In this study, a randomised controlled trial was conducted to evaluate the effect of a web-based multimedia decision aid on decision-making regarding prenatal testing. The decision aid provided both written and audiovisual information on prenatal tests currently available, that is, prenatal screening by first-trimester combined testing, NIPT and invasive diagnostic testing through chorionic villus sampling or amniocentesis. Furthermore, it contained values clarification exercises encouraging pregnant women to reflect on the potential harms and benefits of having prenatal tests performed. The use of the decision aid improved informed decision-making regarding prenatal testing. Of pregnant women allocated to the intervention group (n=130) 82.3% made an informed choice compared with 66.4% of women in the control group (n=131), P=0.004. As the vast majority of pregnant women made decisions consistent with their attitudes towards having prenatal testing performed, this improvement in informed decision-making could be attributed mainly to an increase in decision-relevant knowledge. This study shows that the implementation of a web-based multimedia decision aid directly facilitates the ultimate goal of prenatal testing for fetal chromosomal abnormalities, which is enabling informed autonomous reproductive choice.
Figures
References
-
- Health Council of the Netherlands. Prenatal screening: Down syndrome, neural tube defects, routine ultrasonography. The Hague: Health Council of the Netherlands, 2001; publication no. 2001/11.
-
- De Jong A, Dondorp WJ, Frints SGM, De Die-Smulders CEM, De Wert GMWR: Advances in prenatal screening: the ethical dimension. Nat Rev Genet 2011; 12: 657–663. - PubMed
-
- Michie A, Dormandy E, Marteau TM: The multi-dimensional measure of informed choice: a validation study. Patient Educ Couns 2002; 48: 87–91. - PubMed
-
- Green JM, Hewison J, Bekker HL, Bryant LD, Cuckle HS: Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review. Health Technol Assess 2004; 8: 1–128. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

