Seasonal immunoregulation in a naturally-occurring vertebrate
- PMID: 27189372
- PMCID: PMC4870750
- DOI: 10.1186/s12864-016-2701-7
Seasonal immunoregulation in a naturally-occurring vertebrate
Abstract
Background: Fishes show seasonal patterns of immunity, but such phenomena are imperfectly understood in vertebrates generally, even in humans and mice. As these seasonal patterns may link to infectious disease risk and individual condition, the nature of their control has real practical implications. Here we characterize seasonal dynamics in the expression of conserved vertebrate immunity genes in a naturally-occurring piscine model, the three-spined stickleback.
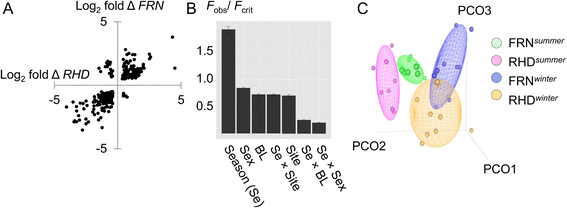
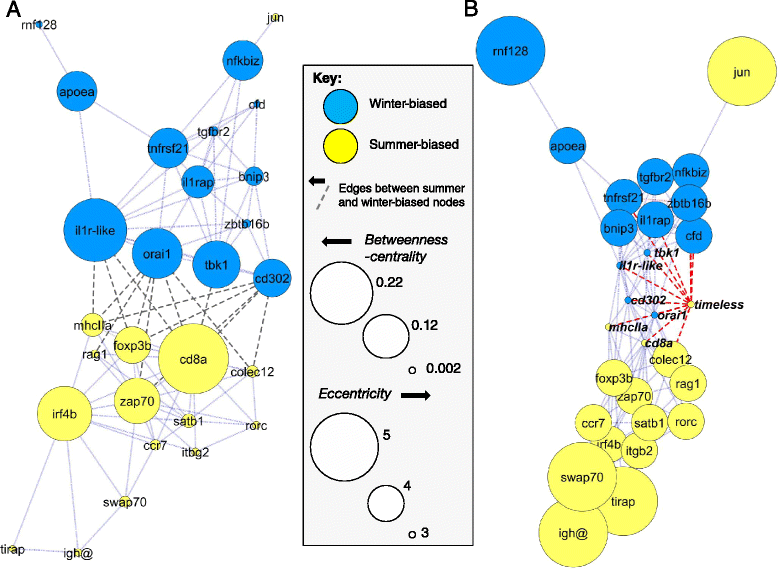
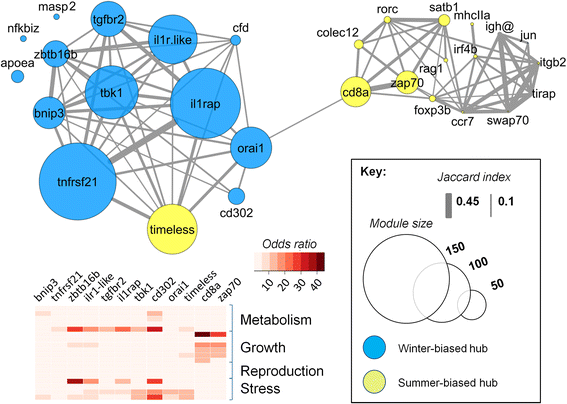
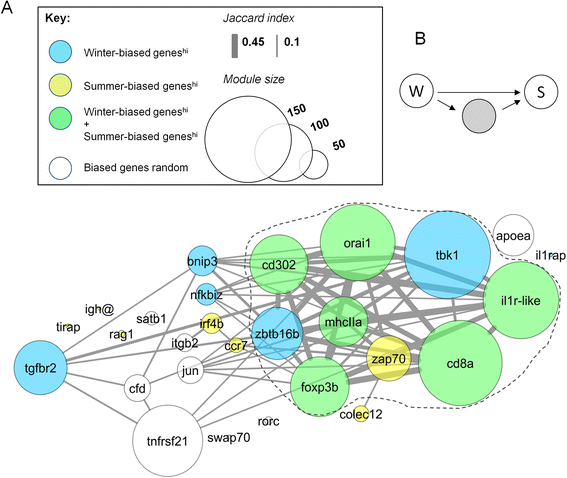
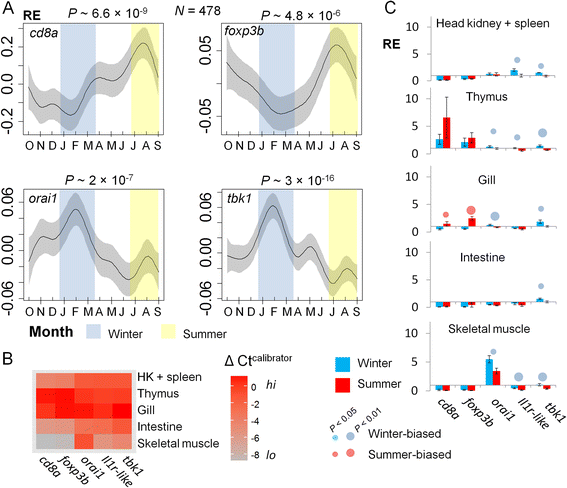
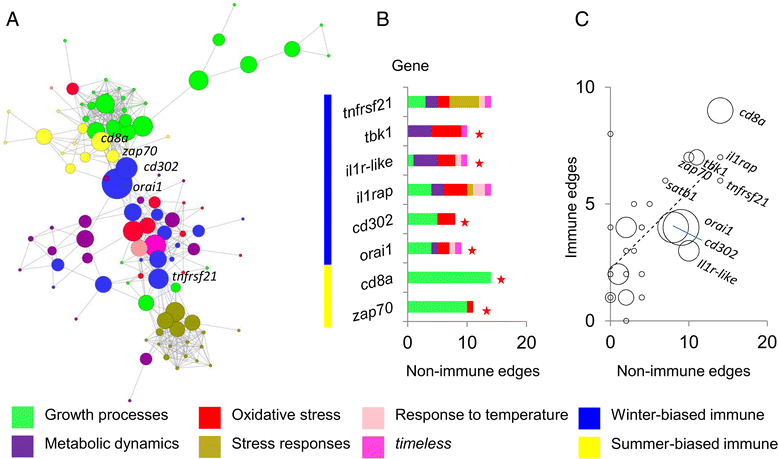
Results: We made genome-wide measurements (RNAseq) of whole-fish mRNA pools (n = 36) at the end of summer and winter in contrasting habitats (riverine and lacustrine) and focussed on common trends to filter habitat-specific from overarching temporal responses. We corroborated this analysis with targeted year-round whole-fish gene expression (Q-PCR) studies in a different year (n = 478). We also considered seasonal tissue-specific expression (6 tissues) (n = 15) at a third contrasting (euryhaline) locality by Q-PCR, further validating the generality of the patterns seen in whole fish analyses. Extremes of season were the dominant predictor of immune expression (compared to sex, ontogeny or habitat). Signatures of adaptive immunity were elevated in late summer. In contrast, late winter was accompanied by signatures of innate immunity (including IL-1 signalling and non-classical complement activity) and modulated toll-like receptor signalling. Negative regulators of T-cell activity were prominent amongst winter-biased genes, suggesting that adaptive immunity is actively down-regulated during winter rather than passively tracking ambient temperature. Network analyses identified a small set of immune genes that might lie close to a regulatory axis. These genes acted as hubs linking summer-biased adaptive pathways, winter-biased innate pathways and other organismal processes, including growth, metabolic dynamics and responses to stress and temperature. Seasonal change was most pronounced in the gill, which contains a considerable concentration of T-cell activity in the stickleback.
Conclusions: Our results suggest major and predictable seasonal re-adjustments of immunity. Further consideration should be given to the effects of such responses in seasonally-occurring disease.
Keywords: Ecoimmunology; Immunity; Immunoregulation; RNAseq; Seasonality; Teleost; Three-spined stickleback; Wildlife.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

