Childhood Pompe disease: clinical spectrum and genotype in 31 patients
- PMID: 27189384
- PMCID: PMC4870771
- DOI: 10.1186/s13023-016-0442-y
Childhood Pompe disease: clinical spectrum and genotype in 31 patients
Abstract
Background: As little information is available on children with non-classic presentations of Pompe disease, we wished to gain knowledge of specific clinical characteristics and genotypes. We included all patients younger than 18 years, who had been evaluated at the Pompe Center in Rotterdam, the Netherlands, between 1975 and 2012, excluding those with the classic-infantile form. None were treated with enzyme replacement therapy at the time of evaluation. We collected information on first symptoms, diagnosis, use of a wheelchair and/or respirator, and enzyme and mutation analysis and assessed muscle strength, pulmonary function, and cardiac parameters.
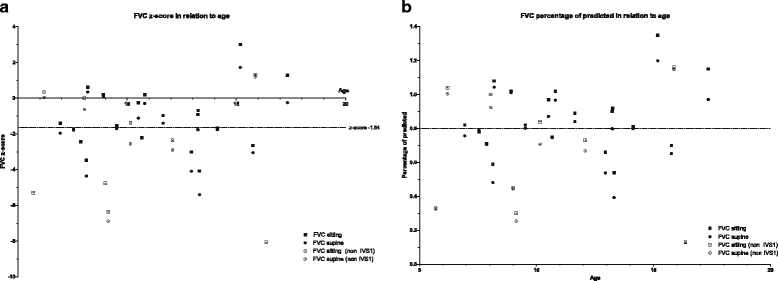
Results: Thirty-one patients participated. Median age at symptom onset was 2.6 years (range 0.5-13y) and at diagnosis 4.0 years. Most first problems were delayed motor development and problems related to limb-girdle weakness. Fatigue, persistent diarrhea and problems in raising the head in supine position were other first complaints. Ten patients were asymptomatic at time of diagnosis. Five of them developed symptoms before inclusion in this study. Over 50 % of all patients had low or absent reflexes, a myopathic face, and scoliosis; 29 % were underweight. Muscle strength of the neck flexors, hip extensors, hip flexors, and shoulder abductors were most frequently reduced. Pulmonary function was decreased in over 48 % of the patients; 2 patients had cardiac hypertrophy. Patients with mutations other than the c.-32-13T > G were overall more severely affected, while 18 out of the 21 patients (86 %) with the c.-32-13T > G/'null' genotype were male.
Conclusions: Our study shows that Pompe disease can present with severe mobility and respiratory problems during childhood. Pompe disease should be considered in the differential diagnosis of children with less familiar signs such as disproportional weakness of the neck flexors, unexplained fatigue, persistent diarrhea and unexplained high CK/ASAT/ALAT. Disease presentation appears to be different from adult patients. The majority of affected children with GAA genotype c.-32-13T > G/'null' appeared to be male.
Keywords: Childhood; Clinical spectrum; Genotype; Natural course; Pompe disease.
Figures
References
-
- Hirschhorn R, Reuser AJJ. Glycogen Storage Disease Type II: acid alpha-glucosidase (acid maltase) deficiency. In: Scriver CR, Beaudet AL, Valle D, Sly WS, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. pp. 3389–420.
-
- Engel AG, Hirschhorn R, Huie ML. Acid maltase deficiency. In: Engel AG, editor. Myology. New York: McGraw-Hill; 2004. pp. 1559–86.
-
- Pompe JC. Over idiopathische hypertrofie van het hart. Ned Tijdsch Geneesk. 1932;76:304–11.
-
- van den Hout HM, Hop W, van Diggelen OP, Smeitink JA, Smit GP, Poll-The BT, Bakker HD, Loonen MC, de Klerk JB, Reuser AJ, et al. The natural course of infantile Pompe’s disease: 20 original cases compared with 133 cases from the literature. Pediatrics. 2003;112(2):332–40. doi: 10.1542/peds.112.2.332. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous