Searching the Evolutionary Origin of Epithelial Mucus Protein Components-Mucins and FCGBP
- PMID: 27189557
- PMCID: PMC4948705
- DOI: 10.1093/molbev/msw066
Searching the Evolutionary Origin of Epithelial Mucus Protein Components-Mucins and FCGBP
Abstract
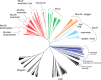
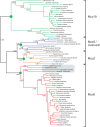
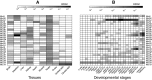
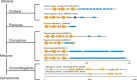
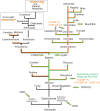
The gel-forming mucins are large glycosylated proteins that are essential components of the mucus layers covering epithelial cells. Using novel methods of identifying mucins based on profile hidden Markov models, we have found a large number of such proteins in Metazoa, aiding in their classification and allowing evolutionary studies. Most vertebrates have 5-6 gel-forming mucin genes and the genomic arrangement of these genes is well conserved throughout vertebrates. An exception is the frog Xenopus tropicalis with an expanded repertoire of at least 26 mucins of this type. Furthermore, we found that the ovomucin protein, originally identified in chicken, is characteristic of reptiles, birds, and amphibians. Muc6 is absent in teleost fish, but we now show that it is present in animals such as ghost sharks, demonstrating an early origin in vertebrate evolution. Public RNA-Seq data were analyzed with respect to mucins in zebrafish, frog, and chicken, thus allowing comparison in regard of tissue and developmental specificity. Analyses of invertebrate proteins reveal that gel-forming-mucin type of proteins is widely distributed also in this group. Their presence in Cnidaria, Porifera, and in Ctenophora (comb jellies) shows that these proteins were present early in metazoan evolution. Finally, we examined the evolution of the FCGBP protein, abundant in mucus and related to gel-forming mucins in terms of structure and localization. We demonstrate that FCGBP, ubiquitous in vertebrates, has a conserved N-terminal domain. Interestingly, this domain is also present as an N-terminal sequence in a number of bacterial proteins.
Keywords: bioinformatics; evolution; mucin; mucus; von Willebrand D domain..
© The Author 2016. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures
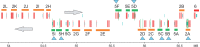


References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol. 215:403–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

