A web resource for mining HLA associations with adverse drug reactions: HLA-ADR
- PMID: 27189608
- PMCID: PMC5647400
- DOI: 10.1093/database/baw069
A web resource for mining HLA associations with adverse drug reactions: HLA-ADR
Abstract
Human leukocyte antigens (HLA) are an important family of genes involved in the immune system. Their primary function is to allow the host immune system to be able to distinguish between self and non-self peptides-e.g. derived from invading pathogens. However, these genes have also been implicated in immune-mediated adverse drug reactions (ADRs), presenting a problem to patients, clinicians and pharmaceutical companies. We have previously developed the Allele Frequency Net Database (AFND) that captures the allelic and haplotype frequencies for these HLA genes across many healthy populations from around the world. Here, we report the development and release of the HLA-ADR database that captures data from publications where HLA alleles and haplotypes have been associated with ADRs (e.g. Stevens-Johnson Syndrome/toxic epidermal necrolysis and drug-induced liver injury). HLA-ADR was created by using data obtained through systematic review of the literature and semi-automated literature mining. The database also draws on data already present in AFND allowing users to compare and analyze allele frequencies in both ADR patients and healthy populations. The HLA-ADR database provides clinicians and researchers with a centralized resource from which to investigate immune-mediated ADRs.Database URL: http://www.allelefrequencies.net/hla-adr/.
© The Author(s) 2016. Published by Oxford University Press.
Figures
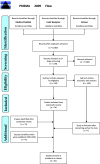



References
-
- Kongkaew C., Hann M., Mandal J. et al. (2013) Risk factors for hospital admissions associated with adverse drug events. Pharmacotherapy, 33, 827–837. - PubMed
-
- Rawlins M., Thompson J. (1977) Pathogenesis of adverse drug reactions David M. Davies. In: Textbook of Adverse Drug Reactions. Oxford: Oxford University Press, p. 10.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

