Asthma
- PMID: 27189668
- PMCID: PMC7096989
- DOI: 10.1038/nrdp.2015.25
Asthma
Abstract
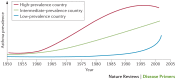
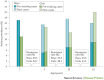
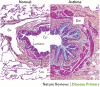
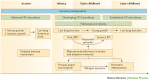
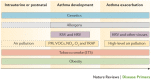
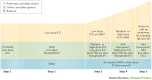
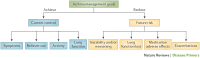
Asthma is the most common inflammatory disease of the lungs. The prevalence of asthma is increasing in many parts of the world that have adopted aspects of the Western lifestyle, and the disease poses a substantial global health and economic burden. Asthma involves both the large-conducting and the small-conducting airways, and is characterized by a combination of inflammation and structural remodelling that might begin in utero. Disease progression occurs in the context of a developmental background in which the postnatal acquisition of asthma is strongly linked with allergic sensitization. Most asthma cases follow a variable course, involving viral-induced wheezing and allergen sensitization, that is associated with various underlying mechanisms (or endotypes) that can differ between individuals. Each set of endotypes, in turn, produces specific asthma characteristics that evolve across the lifecourse of the patient. Strong genetic and environmental drivers of asthma interconnect through novel epigenetic mechanisms that operate prenatally and throughout childhood. Asthma can spontaneously remit or begin de novo in adulthood, and the factors that lead to the emergence and regression of asthma, irrespective of age, are poorly understood. Nonetheless, there is mounting evidence that supports a primary role for structural changes in the airways with asthma acquisition, on which altered innate immune mechanisms and microbiota interactions are superimposed. On the basis of the identification of new causative pathways, the subphenotyping of asthma across the lifecourse of patients is paving the way for more-personalized and precise pathway-specific approaches for the prevention and treatment of asthma, creating the real possibility of total prevention and cure for this chronic inflammatory disease.
Conflict of interest statement
S.T.H. is a non-executive director and a consultant for Synairgen, and a consultant for AstraZeneca and Novartis. He is in receipt of a UK Medical Research Council (MRC) programme grant and receives salary support as an MRC clinical professor. S.W. has served as a consultant for Aerocrine, GlaxoSmithKline and AstraZeneca. She has received research funding (paid to her institution) from GlaxoSmithKline, AstraZeneca, Genentech and Sanofi-Aventis. She has also received research support from the NIH National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID), which provides her salary support. D.S.P. has received an unrestricted educational grant for research (paid to the University of Groningen) from AstraZeneca. Her travel to the European Respiratory Society (ERS) and/or the American Thoracic Society (ATS) meetings has been partially funded by AstraZeneca, Chiesi, GlaxoSmithKline and Takeda. Fees for consultancies were given to the University of Groningen by AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Takeda and Teva. Her travel and lectures in China have been funded by Chiesi. S.T.W. is funded by the NIH NHLBI and is a consultant for the TENOR study for Novartis. H.R. has received research support from the German Research Foundation (DFG), the Federal Ministry of Education and Research (BMBF), the European Union, Land Hessen, the German Academic Exchange Service (DAAD), ALK, Stiftung Pathobiochemie, Ernst-Wendt-Stiftung, Mead Johnson Nutritional and Beckman Coulter. He has also received speakers honoraria from Allergopharma, Novartis, Thermo Fisher Scientific, Danone, Mead Johnson Nutritional and Bencard Allergie. H.R. also serves as a consultant for Bencard Allergie and Sterna Biologicals (for which he is also a cofounder). P.D.S. declares no competing interests.
Figures





References
-
- Global Initiative for Asthma. Global strategy for asthma management and prevention GINA[online], (2015).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

