Caffeine for the Treatment of Apnea in Bronchiolitis: A Randomized Trial
- PMID: 27189681
- PMCID: PMC7126124
- DOI: 10.1016/j.jpeds.2016.04.060
Caffeine for the Treatment of Apnea in Bronchiolitis: A Randomized Trial
Abstract
Objective: To evaluate the efficacy and safety of caffeine citrate in the treatment of apnea in bronchiolitis.
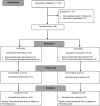
Study design: Eligible infants aged ≤4 months presenting to the main pediatric emergency service with apnea associated bronchiolitis were stratified by gestational age (<34 weeks or longer) and randomized to receive a single dose of intravenous 25 mg/kg caffeine citrate or saline placebo. The primary efficacy outcome was a 24-hour apnea-free period beginning after completion of the blinded study drug infusion. Secondary outcomes were frequency of apnea by 24, 48, and 72 hours after study medication, need for noninvasive/invasive ventilation, and length of stay in the hospital's pediatric intensive care/step-down unit.
Results: A total of 90 infants diagnosed with viral bronchiolitis associated with apnea (median age, 38 days) were enrolled. The rate of respiratory virus panel positivity was similar in the 2 groups (78% for the placebo group vs 84% for the caffeine group). The geometric mean duration to a 24-hour apnea-free period was 28.1 hours (95% CI, 25.6-32.3 hours) for the caffeine group and 29.1 hours (95% CI, 25.7-32.9 hours) for the placebo group (P = .88; OR, 0.99; 95% CI, 0.83-1.17). The frequency of apnea at 24 hours, 24-48 hours, and 48-72 hours after enrollment and the need for noninvasive and invasive ventilation were similar in the 2 groups. No safety issues were reported.
Conclusions: A single dose of caffeine citrate did not significantly reduce apnea episodes associated with bronchiolitis.
Trial registration: Clinicaltrials.gov: NCT01435486.
Keywords: apnea; bronchiolitis; caffeine citrate; respiratory syncytial virus.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Apnea in Bronchiolitis: Challenges of Studying an Uncommon Complication of a Common Condition.J Pediatr. 2016 Oct;177:11-12. doi: 10.1016/j.jpeds.2016.06.038. Epub 2016 Jul 14. J Pediatr. 2016. PMID: 27423173 No abstract available.
-
Caffeine for apnea in bronchiolitis.J Pediatr. 2017 Mar;182:405. doi: 10.1016/j.jpeds.2016.10.047. Epub 2016 Nov 11. J Pediatr. 2017. PMID: 27839700 No abstract available.
-
Reply.J Pediatr. 2017 Mar;182:405-406. doi: 10.1016/j.jpeds.2016.10.048. Epub 2016 Nov 18. J Pediatr. 2017. PMID: 27871689 No abstract available.
References
-
- Koehoorn M., Karr C.J., Demers P.A., Lencar C., Tamburic L., Brauer M. Descriptive epidemiological features of bronchiolitis in a population-based cohort. Pediatrics. 2008;122:1196–1203. - PubMed
-
- Jafri H.S., Wu X., Makari D., Henrickson K.J. Distribution of respiratory syncytial virus subtypes A and B among infants presenting to the emergency department with lower respiratory tract infection or apnea. Pediatr Infect Dis J. 2013;32:335–340. - PubMed
-
- Staat M.A., Henrickson K., Elhefni H., Groothuis J., Makari D. Prevalence of respiratory syncytial virus-associated lower respiratory infection and apnea in infants presenting to the emergency department. Pediatr Infect Dis J. 2013;32:911–914. - PubMed
-
- Pappano D.A., Bass E.S. Respiratory virus-associated apnea. Pediatr Emerg Med Rep. 2007;12:1–12.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

