Neuronal activity mediated regulation of glutamate transporter GLT-1 surface diffusion in rat astrocytes in dissociated and slice cultures
- PMID: 27189737
- PMCID: PMC4915597
- DOI: 10.1002/glia.22997
Neuronal activity mediated regulation of glutamate transporter GLT-1 surface diffusion in rat astrocytes in dissociated and slice cultures
Abstract
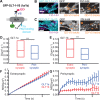
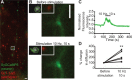
The astrocytic GLT-1 (or EAAT2) is the major glutamate transporter for clearing synaptic glutamate. While the diffusion dynamics of neurotransmitter receptors at the neuronal surface are well understood, far less is known regarding the surface trafficking of transporters in subcellular domains of the astrocyte membrane. Here, we have used live-cell imaging to study the mechanisms regulating GLT-1 surface diffusion in astrocytes in dissociated and brain slice cultures. Using GFP-time lapse imaging, we show that GLT-1 forms stable clusters that are dispersed rapidly and reversibly upon glutamate treatment in a transporter activity-dependent manner. Fluorescence recovery after photobleaching and single particle tracking using quantum dots revealed that clustered GLT-1 is more stable than diffuse GLT-1 and that glutamate increases GLT-1 surface diffusion in the astrocyte membrane. Interestingly, the two main GLT-1 isoforms expressed in the brain, GLT-1a and GLT-1b, are both found to be stabilized opposed to synapses under basal conditions, with GLT-1b more so. GLT-1 surface mobility is increased in proximity to activated synapses and alterations of neuronal activity can bidirectionally modulate the dynamics of both GLT-1 isoforms. Altogether, these data reveal that astrocytic GLT-1 surface mobility, via its transport activity, is modulated during neuronal firing, which may be a key process for shaping glutamate clearance and glutamatergic synaptic transmission. GLIA 2016;64:1252-1264.
Keywords: neuron-astrocyte interaction; organotypic slices; single particle tracking; synapse.
© 2016 The Authors. Glia Published by Wiley Periodicals, Inc.
Figures



References
-
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderón NC, Esposti F, Borghuis BG, Sun XR, Gordus A, Orger MB, Portugues R, Engert F, Macklin JJ, Filosa A, Aggarwal A, Kerr RA, Takagi R, Kracun S, Shigetomi E, Khakh BS, Baier H, Lagnado L, Wang SS, Bargmann CI, Kimmel BE, Jayaraman V, Svoboda K, Kim DS, Schreiter ER, Looger LL. 2012. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci 32:13819–13840. - PMC - PubMed
-
- Asztely F, Erdemli G, Kullmann DM. 1997. Extrasynaptic glutamate spillover in the hippocampus: Dependence on temperature and the role of active glutamate uptake. Neuron 18:281–293. - PubMed
-
- Attwell D, Barbour B, Szatkowski M. 1993. Nonvesicular release of neurotransmitter. Neuron 11:401–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

