Rhodium(I)-Catalyzed Benzannulation of Heteroaryl Propargylic Esters: Synthesis of Indoles and Related Heterocycles
- PMID: 27189811
- PMCID: PMC4945426
- DOI: 10.1002/chem.201602088
Rhodium(I)-Catalyzed Benzannulation of Heteroaryl Propargylic Esters: Synthesis of Indoles and Related Heterocycles
Abstract
A de novo synthesis of a benzene ring allows for the preparation of a diverse range of heterocycles including indoles, benzofurans, benzothiophenes, carbazoles, and dibenzofurans from simple heteroaryl propargylic esters using a unified carbonylative benzannulation strategy. Multiple substituents can be easily introduced to the C4-C7 positions of indoles and related heterocycles.
Keywords: annulation; carbonylation; catalysis; indoles; rhodium.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


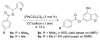


Similar articles
-
Synthesis of Carbazoles and Carbazole-Containing Heterocycles via Rhodium-Catalyzed Tandem Carbonylative Benzannulations.J Org Chem. 2016 Apr 1;81(7):2930-42. doi: 10.1021/acs.joc.6b00212. Epub 2016 Mar 21. J Org Chem. 2016. PMID: 26963834
-
[4 + 2] Benzannulation of 3-Alkenylpyrroles/Thiophenes with Propargylic Alcohols: Access to Substituted Indoles, Benzothiophenes, and Aza[5]helicenes.J Org Chem. 2017 Mar 3;82(5):2345-2354. doi: 10.1021/acs.joc.6b02637. Epub 2017 Feb 22. J Org Chem. 2017. PMID: 28195471
-
Rhodium enalcarbenoids: direct synthesis of indoles by rhodium(II)-catalyzed [4+2] benzannulation of pyrroles.Angew Chem Int Ed Engl. 2014 Apr 14;53(16):4076-80. doi: 10.1002/anie.201400161. Epub 2014 Mar 3. Angew Chem Int Ed Engl. 2014. PMID: 24590818
-
One-Pot Sequential Propargylation/Cycloisomerization: A Facile [4+2]-Benzannulation Approach to Carbazoles.Chemistry. 2016 Feb 12;22(7):2501-6. doi: 10.1002/chem.201503496. Epub 2016 Jan 14. Chemistry. 2016. PMID: 26836577
-
Synthesis of functionalized indoles via cascade benzannulation strategies: a decade's overview.Org Biomol Chem. 2022 Apr 13;20(15):3029-3042. doi: 10.1039/d2ob00187j. Org Biomol Chem. 2022. PMID: 35332905 Review.
Cited by
-
Carbonylative synthesis and functionalization of indoles.Beilstein J Org Chem. 2024 Apr 30;20:973-1000. doi: 10.3762/bjoc.20.87. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38711593 Free PMC article. Review.
-
Rhodium-Catalyzed (5 + 2) and (5 + 1) Cycloadditions Using 1,4-Enynes as Five-Carbon Building Blocks.Acc Chem Res. 2020 Jan 21;53(1):231-243. doi: 10.1021/acs.accounts.9b00477. Epub 2019 Dec 10. Acc Chem Res. 2020. PMID: 31820914 Free PMC article. Review.
References
-
-
For selected reviews on indole synthesis, see: Barluenga J, Rodriguez F, Fananas FJ. Chem Asian J. 2009;4:1036.Taber DF, Tirunahari PK. Tetrahedron. 2011;67:7195.Vicente R. Org Biomol Chem. 2011;9:6469.Platon M, Amardeil R, Djakovitch L, Hierso JC. Chem Soc Rev. 2012;41:3929.
-
-
-
For a leading reference on de novo synthesis of polysubstituted benzenes, see: Izawa Y, Pun D, Stahl SS. Science. 2011;333:209.For a recent example on making indoles by benzannulation, see: Lam TY, Wang Y-P, Danheiser RL. J Org Chem. 2013;78:9396.
-
-
-
Dötz KH. Angew Chem Int Ed. 1975;14:644.Dötz KH. Angew Chem Int Ed. 1984;23:587.Wulff WD, Tang PC, Chan KS, McCallum JS, Yang DC, Gilbertson SR. Tetrahedron. 1985;41:5813.
-
-
-
For recent reviews on reactions involving Fischer carbenes, see: Gomez-Gallego M, Mancheno MJ, Sierra MA. Acc Chem Res. 2005;38:44.Dötz KH, Stendel J., Jr Chem Rev. 2009;109:3227.
-
-
- Wulf WD. In: Advances in Metal-Organic Chemistry. Liebeskind LS, editor. Vol. 1. JAI Press; Greenwich, CT: 1989. p. 336.
- Lian YQ, Wulff WD. J Am Chem Soc. 2005;127:17162. - PMC - PubMed
- Merlic CA, Xu DQ. J Am Chem Soc. 1991;113:7418.
- Merlic CA, Burns EE, Xu DQ, Chen SY. J Am Chem Soc. 1992;114:8722.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

