Cross-linked Composite Gel Polymer Electrolyte using Mesoporous Methacrylate-Functionalized SiO2 Nanoparticles for Lithium-Ion Polymer Batteries
- PMID: 27189842
- PMCID: PMC4870681
- DOI: 10.1038/srep26332
Cross-linked Composite Gel Polymer Electrolyte using Mesoporous Methacrylate-Functionalized SiO2 Nanoparticles for Lithium-Ion Polymer Batteries
Abstract
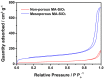
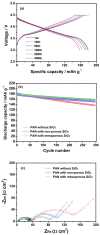
Liquid electrolytes composed of lithium salt in a mixture of organic solvents have been widely used for lithium-ion batteries. However, the high flammability of the organic solvents can lead to thermal runaway and explosions if the system is accidentally subjected to a short circuit or experiences local overheating. In this work, a cross-linked composite gel polymer electrolyte was prepared and applied to lithium-ion polymer cells as a safer and more reliable electrolyte. Mesoporous SiO2 nanoparticles containing reactive methacrylate groups as cross-linking sites were synthesized and dispersed into the fibrous polyacrylonitrile membrane. They directly reacted with gel electrolyte precursors containing tri(ethylene glycol) diacrylate, resulting in the formation of a cross-linked composite gel polymer electrolyte with high ionic conductivity and favorable interfacial characteristics. The mesoporous SiO2 particles also served as HF scavengers to reduce the HF content in the electrolyte at high temperature. As a result, the cycling performance of the lithium-ion polymer cells with cross-linked composite gel polymer electrolytes employing methacrylate-functionalized mesoporous SiO2 nanoparticles was remarkably improved at elevated temperatures.
Figures






References
-
- Tarascon J.-M. & Armand M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001). - PubMed
-
- Xu K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4417 (2004). - PubMed
-
- Aurbach D. et al. Design of electrolyte solutions for Li and Li-ion batteries: a review. Electrochim. Acta 50, 247–254 (2004).
-
- Armand M., Endres F., MacFarlane D. R., Ohno H. & Scrosati B. Ionic-liquid materials for the electrochemical challenges of the future. Nature Mater. 8, 621–629 (2009). - PubMed
-
- Marom R., Amalraj S. F., Leifer N., Jacob D. & Aurbach D. A review of advanced and practical lithium battery materials. J. Mater. Chem. 21, 9938–9954 (2011).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

