Insights into the role of neuronal glucokinase
- PMID: 27189932
- PMCID: PMC4967152
- DOI: 10.1152/ajpendo.00034.2016
Insights into the role of neuronal glucokinase
Abstract
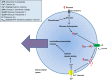
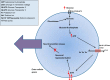
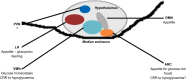
Glucokinase is a key component of the neuronal glucose-sensing mechanism and is expressed in brain regions that control a range of homeostatic processes. In this review, we detail recently identified roles for neuronal glucokinase in glucose homeostasis and counterregulatory responses to hypoglycemia and in regulating appetite. We describe clinical implications from these advances in our knowledge, especially for developing novel treatments for diabetes and obesity. Further research required to extend our knowledge and help our efforts to tackle the diabetes and obesity epidemics is suggested.
Keywords: appetite; counterregulatory response; glucokinase; glucose homeostasis; glucose sensing; neuronal.
Copyright © 2016 the American Physiological Society.
Figures

References
-
- Adachi A, Kobashi M. Chemosensitive neurons within the area postrema of the rat. Neurosci Lett 55: 137–140, 1985. - PubMed
-
- Adachi A, Kobashi M, Funahashi M. Glucose-responsive neurons in the brainstem. Obes Res 3, Suppl 5: 735s–740s, 1995. - PubMed
-
- Adachi A, Kobashi M, Miyoshi N, Tsukamoto G. Chemosensitive neurons in the area postrema of the rat and their possible functions. Brain Res Bull 26: 137–140, 1991. - PubMed
-
- Ahren B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia 43: 393–410, 2000. - PubMed
-
- Alvarez E, Martinez MD, Roncero I, Chowen JA, Garcia-Cuartero B, Gispert JD, Sanz C, Vazquez P, Maldonado A, De Caceres J, Desco M, Pozo MA, Blazquez E. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem 92: 798–806, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 090792/Z/09/A/WT_/Wellcome Trust/United Kingdom
- BB/I00842X /BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/I00842X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MRC_/Medical Research Council/United Kingdom
- BB/E52708X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

