Thiopurine Prodrugs Mediate Immunosuppressive Effects by Interfering with Rac1 Protein Function
- PMID: 27189938
- PMCID: PMC4919453
- DOI: 10.1074/jbc.M115.694422
Thiopurine Prodrugs Mediate Immunosuppressive Effects by Interfering with Rac1 Protein Function
Abstract
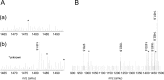
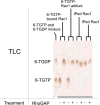
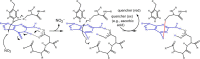
6-Thiopurine (6-TP) prodrugs include 6-thioguanine and azathioprine. Both are widely used to treat autoimmune disorders and certain cancers. This study showed that a 6-thioguanosine triphosphate (6-TGTP), converted in T-cells from 6-TP, targets Rac1 to form a disulfide adduct between 6-TGTP and the redox-sensitive GXXXXGK(S/T)C motif of Rac1. This study also showed that, despite the conservation of the catalytic activity of RhoGAP (Rho-specific GAP) on the 6-TGTP-Rac1 adduct to produce the biologically inactive 6-thioguanosine diphosphate (6-TGDP)-Rac1 adduct, RhoGEF (Rho-specific GEF) cannot exchange the 6-TGDP adducted on Rac1 with free guanine nucleotide. The biologically inactive 6-TGDP-Rac1 adduct accumulates in cells because of the ongoing combined actions of RhoGEF and RhoGAP. Because other Rho GTPases, such as RhoA and Cdc42, also possess the GXXXXGK(S/T)C motif, the proposed mechanism for the inactivation of Rac1 also applies to RhoA and Cdc42. However, previous studies have shown that CD3/CD28-stimulated T-cells contain more activated Rac1 than other Rho GTPases such as RhoA and Cdc42. Accordingly, Rac1 is the main target of 6-TP in activated T-cells. This explains the T-cell-specific Rac1-targeting therapeutic action of 6-TP that suppresses the immune response. This proposed mechanism for the action of 6-TP on Rac1 performs a critical role in demonstrating the capability to design a Rac1-targeting chemotherapeutic agent(s) for autoimmune disorders. Nevertheless, the results also suggest that the targeting action of other Rho GTPases in other organ cells, such as RhoA in vascular cells, may be linked to cytotoxicities because RhoA plays a key role in vasculature functions.
Keywords: 6-TGNP-Rac1 adduct; CD4+ cells; Rac1 inactivation; Ras-related C3 botulinum toxin substrate 1 (Rac1); T-cell; disulfide; immunosuppression; redox regulation; redox-sensitive GXXXXGK(S/T)C motif; thiopurine prodrugs.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures








Similar articles
-
Insight into the 6-thiopurine-mediated termination of the invasive motility of tumor cells derived from inflammatory breast cancer.Biochemistry. 2011 Jun 28;50(25):5731-42. doi: 10.1021/bi200347y. Epub 2011 Jun 2. Biochemistry. 2011. PMID: 21568348
-
Cdc42 and Rac1 activity is reduced in human pheochromocytoma and correlates with FARP1 and ARHGEF1 expression.Endocr Relat Cancer. 2016 Apr;23(4):281-93. doi: 10.1530/ERC-15-0502. Epub 2016 Feb 24. Endocr Relat Cancer. 2016. PMID: 26911374
-
Isoform-specific roles of the GTPase activating protein Nadrin in cytoskeletal reorganization of platelets.Cell Signal. 2013 Jan;25(1):236-46. doi: 10.1016/j.cellsig.2012.09.005. Epub 2012 Sep 10. Cell Signal. 2013. PMID: 22975681
-
The RHO Family GTPases: Mechanisms of Regulation and Signaling.Cells. 2021 Jul 20;10(7):1831. doi: 10.3390/cells10071831. Cells. 2021. PMID: 34359999 Free PMC article. Review.
-
The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review.
Cited by
-
Treatment of Inflammatory Bowel Disease: A Comprehensive Review.Front Med (Lausanne). 2021 Dec 20;8:765474. doi: 10.3389/fmed.2021.765474. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34988090 Free PMC article. Review.
-
Azathioprine with Allopurinol: Lower Deoxythioguanosine in DNA and Transcriptome Changes Indicate Mechanistic Differences to Azathioprine Alone.Inflamm Bowel Dis. 2017 Jun;23(6):946-955. doi: 10.1097/MIB.0000000000001131. Inflamm Bowel Dis. 2017. PMID: 28452864 Free PMC article.
-
Cheese starter cultures attenuate inflammation in the in vitro Caco-2 model.AIMS Microbiol. 2025 May 28;11(2):369-387. doi: 10.3934/microbiol.2025017. eCollection 2025. AIMS Microbiol. 2025. PMID: 40600210 Free PMC article.
-
Mosaic Tetrasomy 9p Associated With Inflammatory Bowel Disease.J Crohns Colitis. 2019 Oct 28;13(11):1474-1478. doi: 10.1093/ecco-jcc/jjz079. J Crohns Colitis. 2019. PMID: 31104071 Free PMC article.
-
Pharmacological targeting of allergen-specific T lymphocytes.Immunol Lett. 2017 Sep;189:27-39. doi: 10.1016/j.imlet.2017.03.010. Epub 2017 Mar 18. Immunol Lett. 2017. PMID: 28322861 Free PMC article. Review.
References
-
- Langmuir P. B., Aplenc R., and Lange B. J. (2001) Acute myeloid leukaemia in children. Best Pract. Res. Clin. Haematol. 14, 77–93 - PubMed
-
- Elion G. B. (1989) The purine path to chemotherapy. Science 244, 41–47 - PubMed
-
- Gearry R. B., and Barclay M. L. (2005) Azathioprine and 6-mercaptopurine pharmacogenetics and metabolite monitoring in inflammatory bowel disease. J. Gastroenterol. Hepatol. 20, 1149–1157 - PubMed
-
- McDonald E. R. 3rd., Wu G. S., Waldman T., and El-Deiry W. S. (1996) Repair defect in p21 WAF1/CIP1−/− human cancer cells. Cancer Res. 56, 2250–2255 - PubMed
-
- Tiede I., Fritz G., Strand S., Poppe D., Dvorsky R., Strand D., Lehr H. A., Wirtz S., Becker C., Atreya R., Mudter J., Hildner K., Bartsch B., Holtmann M., Blumberg R., et al. (2003) CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J. Clin. Invest. 111, 1133–1145 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

