Reactive Oxygen Species Differentially Regulate Bone Turnover in an Age-Specific Manner in Catalase Transgenic Female Mice
- PMID: 27189961
- PMCID: PMC4931876
- DOI: 10.1124/jpet.116.233213
Reactive Oxygen Species Differentially Regulate Bone Turnover in an Age-Specific Manner in Catalase Transgenic Female Mice
Abstract
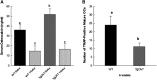
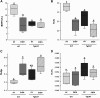
Chronic ethyl alcohol (EtOH) consumption results in reactive oxygen species (ROS) generation in bone and osteopenia due to increased bone resorption and reduced bone formation. In this study, transgenic C57Bl/6J mice overexpressing human catalase (TgCAT) were used to test whether limiting excess hydrogen peroxide would protect against EtOH-mediated bone loss. Micro-computed tomography analysis of the skeletons of 6-week-old female chow-fed TgCAT mice revealed a high bone mass phenotype with increased cortical bone area and thickness as well as significantly increased trabecular bone volume (P < 0.05). Six-week-old wild-type (WT) and TgCAT female mice were chow fed or pair fed (PF) liquid diets with or without EtOH, approximately 30% of calories, for 8 weeks. Pair feeding of WT had no demonstrable effect on the skeleton; however, EtOH feeding of WT mice significantly reduced cortical and trabecular bone parameters along with bone strength compared with PF controls (P < 0.05). In contrast, EtOH feeding of TgCAT mice had no effect on trabecular bone compared with PF controls. At 14 weeks of age, there was significantly less trabecular bone and cortical cross-sectional area in TgCAT mice than WT mice (P < 0.05), suggesting impaired normal bone accrual with age. TgCAT mice expressed less collagen1α and higher sclerostin mRNA (P < 0.05), suggesting decreased bone formation in TgCAT mice. In conclusion, catalase overexpression resulted in greater bone mass than in WT mice at 6 weeks and lower bone mass at 14 weeks. EtOH feeding induced significant reductions in bone architecture and strength in WT mice, but TgCAT mice were partially protected. These data implicate ROS signaling in the regulation of bone turnover in an age-dependent manner, and indicate that excess hydrogen peroxide generation contributes to alcohol-induced osteopenia.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures






References
-
- Almeida M, Han L, Martin-Millan M, O’Brien CA, Manolagas SC. (2007) Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem 282:27298–27305. - PubMed
-
- Badger TM, Ronis MJ, Lumpkin CK, Valentine CR, Shahare M, Irby D, Huang J, Mercado C, Thomas P, Ingelman-Sundberg M, et al. (1993) Effects of chronic ethanol on growth hormone secretion and hepatic cytochrome P450 isozymes of the rat. J Pharmacol Exp Ther 264:438–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

