Nuclear Factor Erythroid 2-Related Factor 2 Deficiency Results in Amplification of the Liver Fat-Lowering Effect of Estrogen
- PMID: 27189962
- PMCID: PMC4931875
- DOI: 10.1124/jpet.115.231316
Nuclear Factor Erythroid 2-Related Factor 2 Deficiency Results in Amplification of the Liver Fat-Lowering Effect of Estrogen
Abstract
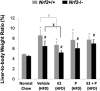
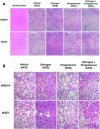
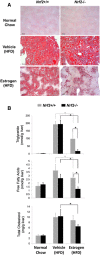
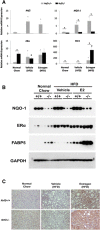
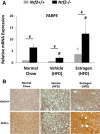
Transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) regulates multiple biologic processes, including hepatic lipid metabolism. Estrogen exerts actions affecting energy homeostasis, including a liver fat-lowering effect. Increasing evidence indicates the crosstalk between these two molecules. The aim of this study was to evaluate whether Nrf2 modulates estrogen signaling in hepatic lipid metabolism. Nonalcoholic fatty liver disease (NAFLD) was induced in wild-type and Nrf2-null mice fed a high-fat diet and the liver fat-lowering effect of exogenous estrogen was subsequently assessed. We found that exogenous estrogen eliminated 49% and 90% of hepatic triglycerides in wild-type and Nrf2-null mice with NAFLD, respectively. This observation demonstrates that Nrf2 signaling is antagonistic to estrogen signaling in hepatic fat metabolism; thus, Nrf2 absence results in striking amplification of the liver fat-lowering effect of estrogen. In addition, we found the association of trefoil factor 3 and fatty acid binding protein 5 with the liver fat-lowering effect of estrogen. In summary, we identified Nrf2 as a novel and potent inhibitor of estrogen signaling in hepatic lipid metabolism. Our finding may provide a potential strategy to treat NAFLD by dually targeting Nrf2 and estrogen signaling.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
Similar articles
-
Niga-ichigoside F1 ameliorates high-fat diet-induced hepatic steatosis in male mice by Nrf2 activation.Food Funct. 2018 Feb 21;9(2):906-916. doi: 10.1039/c7fo01051f. Food Funct. 2018. PMID: 29309075
-
Nrf2 deletion causes "benign" simple steatosis to develop into nonalcoholic steatohepatitis in mice fed a high-fat diet.Lipids Health Dis. 2013 Nov 4;12:165. doi: 10.1186/1476-511X-12-165. Lipids Health Dis. 2013. PMID: 24188280 Free PMC article.
-
Green tea extract provides extensive Nrf2-independent protection against lipid accumulation and NFκB pro- inflammatory responses during nonalcoholic steatohepatitis in mice fed a high-fat diet.Mol Nutr Food Res. 2016 Apr;60(4):858-70. doi: 10.1002/mnfr.201500814. Epub 2016 Feb 18. Mol Nutr Food Res. 2016. PMID: 26679056 Free PMC article.
-
Asperuloside activates hepatic NRF2 signaling to stimulate mitochondrial metabolism and restore lipid homeostasis in high fat diet-induced MAFLD.Eur J Pharmacol. 2024 Nov 15;983:177003. doi: 10.1016/j.ejphar.2024.177003. Epub 2024 Sep 14. Eur J Pharmacol. 2024. PMID: 39278309
-
The Dual Role of Nrf2 in Nonalcoholic Fatty Liver Disease: Regulation of Antioxidant Defenses and Hepatic Lipid Metabolism.Biomed Res Int. 2015;2015:597134. doi: 10.1155/2015/597134. Epub 2015 May 18. Biomed Res Int. 2015. PMID: 26120584 Free PMC article. Review.
Cited by
-
The impact of parity on the risk of nonalcoholic fatty liver disease defined by hepatic steatosis index: A nationwide cohort study.Sci Rep. 2025 Jul 24;15(1):26972. doi: 10.1038/s41598-025-11976-x. Sci Rep. 2025. PMID: 40707536 Free PMC article.
-
Baicalein Attenuates Neuroinflammation by Inhibiting NLRP3/caspase-1/GSDMD Pathway in MPTP Induced Mice Model of Parkinson's Disease.Int J Neuropsychopharmacol. 2020 Aug 6;23(11):762-73. doi: 10.1093/ijnp/pyaa060. Online ahead of print. Int J Neuropsychopharmacol. 2020. PMID: 32761175 Free PMC article.
-
Esculetin Alleviates Nonalcoholic Fatty Liver Disease on High-Cholesterol-Diet-Induced Larval Zebrafish and FFA-Induced BRL-3A Hepatocyte.Int J Mol Sci. 2023 Jan 13;24(2):1593. doi: 10.3390/ijms24021593. Int J Mol Sci. 2023. PMID: 36675107 Free PMC article.
-
Lipid Modulating Anti-oxidant Stress Activity of Gastrodin on Nonalcoholic Fatty Liver Disease Larval Zebrafish Model.Int J Mol Sci. 2019 Apr 23;20(8):1984. doi: 10.3390/ijms20081984. Int J Mol Sci. 2019. PMID: 31018538 Free PMC article.
-
NRF2 deficiency increases obesity susceptibility in a mouse menopausal model.PLoS One. 2020 Feb 11;15(2):e0228559. doi: 10.1371/journal.pone.0228559. eCollection 2020. PLoS One. 2020. PMID: 32045430 Free PMC article.
References
-
- Ansell PJ, Espinosa-Nicholas C, Curran EM, Judy BM, Philips BJ, Hannink M, Lubahn DB. (2004) In vitro and in vivo regulation of antioxidant response element-dependent gene expression by estrogens. Endocrinology 145:311–317. - PubMed
-
- Ansell PJ, Lo SC, Newton LG, Espinosa-Nicholas C, Zhang DD, Liu JH, Hannink M, Lubahn DB. (2005) Repression of cancer protective genes by 17beta-estradiol: ligand-dependent interaction between human Nrf2 and estrogen receptor alpha. Mol Cell Endocrinol 243:27–34. - PubMed
-
- Barros RP, Gustafsson JA. (2011) Estrogen receptors and the metabolic network. Cell Metab 14:289–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

