Preferential Delivery of an Opioid Antagonist to the Fetal Brain in Pregnant Mice
- PMID: 27189967
- PMCID: PMC4931874
- DOI: 10.1124/jpet.115.231902
Preferential Delivery of an Opioid Antagonist to the Fetal Brain in Pregnant Mice
Abstract
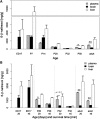
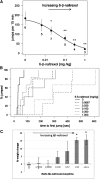
Prolonged fetal exposure to opioids results in neonatal abstinence syndrome (NAS), a major medical problem requiring intensive care and increased hospitalization times for newborns with NAS. Multiple strategies are currently available to alleviate withdrawal in infants with NAS. To prevent NAS caused by opioid maintenance programs in pregnant women, blocking fetal dependence without compromising the mother's opiate therapy is desirable. Here we tested in pregnant mice whether a peripherally selective opioid antagonist can preferentially enter the fetal brain and, thereby, in principle, selectively protect the fetus. We show using mass spectrometry that 6β-naltrexol, a neutral opioid antagonist with very limited ability to cross the blood-brain barrier (BBB), readily crosses the placental barrier and enters the fetal brain at high levels, although it is relatively excluded from the maternal brain. Furthermore, owing to the late development of the BBB in postnatal mice, we show that 6β-naltrexol can readily enter the juvenile mouse brain until at least postnatal day 14. Taking advantage of this observation, we show that long-term exposure to morphine starting in the second postnatal week causes robust and quantifiable dependence behaviors that are suppressed by concomitant administration of 6β-naltrexol with much greater potency (ID50 0.022-0.044 mg/kg, or 1/500 the applied dose of morphine) than previously demonstrated for either the suppression of central nervous system opioid effects or the induction of withdrawal in adults. These results indicate that peripherally selective opioid antagonists capable of penetrating the placenta may be beneficial for preventing or reducing neonatal dependence and NAS in a dose range that should not interfere with maternal opioid maintenance.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures

References
-
- Barr GA, Zmitrovich A, Hamowy AS, Liu PY, Wang S, Hutchings DE. (1998) Neonatal withdrawal following pre- and postnatal exposure to methadone in the rat. Pharmacol Biochem Behav 60:97–104. - PubMed
-
- Chey WD, Webster L, Sostek M, Lappalainen J, Barker PN, Tack J. (2014) Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med 370:2387–2396. - PubMed
-
- Clancy B, Darlington RB, Finlay BL. (2001) Translating developmental time across mammalian species. Neuroscience 105:7–17. - PubMed
-
- Dryden C, Young D, Hepburn M, Mactier H. (2009) Maternal methadone use in pregnancy: factors associated with the development of neonatal abstinence syndrome and implications for healthcare resources. BJOG 116:665–671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

