Cyclooxygenase inhibition targets neurons to prevent early behavioural decline in Alzheimer's disease model mice
- PMID: 27190010
- PMCID: PMC4939702
- DOI: 10.1093/brain/aww117
Cyclooxygenase inhibition targets neurons to prevent early behavioural decline in Alzheimer's disease model mice
Abstract
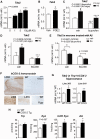
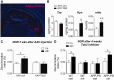
Identifying preventive targets for Alzheimer's disease is a central challenge of modern medicine. Non-steroidal anti-inflammatory drugs, which inhibit the cyclooxygenase enzymes COX-1 and COX-2, reduce the risk of developing Alzheimer's disease in normal ageing populations. This preventive effect coincides with an extended preclinical phase that spans years to decades before onset of cognitive decline. In the brain, COX-2 is induced in neurons in response to excitatory synaptic activity and in glial cells in response to inflammation. To identify mechanisms underlying prevention of cognitive decline by anti-inflammatory drugs, we first identified an early object memory deficit in APPSwe-PS1ΔE9 mice that preceded previously identified spatial memory deficits in this model. We modelled prevention of this memory deficit with ibuprofen, and found that ibuprofen prevented memory impairment without producing any measurable changes in amyloid-β accumulation or glial inflammation. Instead, ibuprofen modulated hippocampal gene expression in pathways involved in neuronal plasticity and increased levels of norepinephrine and dopamine. The gene most highly downregulated by ibuprofen was neuronal tryptophan 2,3-dioxygenase (Tdo2), which encodes an enzyme that metabolizes tryptophan to kynurenine. TDO2 expression was increased by neuronal COX-2 activity, and overexpression of hippocampal TDO2 produced behavioural deficits. Moreover, pharmacological TDO2 inhibition prevented behavioural deficits in APPSwe-PS1ΔE9 mice. Taken together, these data demonstrate broad effects of cyclooxygenase inhibition on multiple neuronal pathways that counteract the neurotoxic effects of early accumulating amyloid-β oligomers.
Keywords: Alzheimer’s disease; cyclooxgenases; hippocampus; ibuprofen; kynurenine pathway.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures







Similar articles
-
Cyclooxygenase-2 inhibition improves amyloid-beta-mediated suppression of memory and synaptic plasticity.Brain. 2008 Mar;131(Pt 3):651-64. doi: 10.1093/brain/awn008. Brain. 2008. PMID: 18292081 Free PMC article.
-
Cycloxygenase-2 activity promotes cognitive deficits but not increased amyloid burden in a model of Alzheimer's disease in a sex-dimorphic pattern.Neuroscience. 2006 Sep 1;141(3):1149-62. doi: 10.1016/j.neuroscience.2006.05.001. Epub 2006 Jun 6. Neuroscience. 2006. PMID: 16753269
-
Social and contextual memory impairments induced by Amyloid-β oligomers are rescued by Sigma-1 receptor activation.Biomed Pharmacother. 2025 Mar;184:117914. doi: 10.1016/j.biopha.2025.117914. Epub 2025 Feb 24. Biomed Pharmacother. 2025. PMID: 39999645
-
Chronic phosphodiesterase type 2 inhibition improves memory in the APPswe/PS1dE9 mouse model of Alzheimer's disease.Neuropharmacology. 2013 Jan;64:124-36. doi: 10.1016/j.neuropharm.2012.06.048. Epub 2012 Jul 4. Neuropharmacology. 2013. PMID: 22771768
-
Blocking the Interaction between EphB2 and ADDLs by a Small Peptide Rescues Impaired Synaptic Plasticity and Memory Deficits in a Mouse Model of Alzheimer's Disease.J Neurosci. 2016 Nov 23;36(47):11959-11973. doi: 10.1523/JNEUROSCI.1327-16.2016. J Neurosci. 2016. PMID: 27881781 Free PMC article.
Cited by
-
Suppression of behavioral activity and hippocampal noradrenaline caused by surgical stress in type 2 diabetes model mice.BMC Neurosci. 2020 Feb 17;21(1):8. doi: 10.1186/s12868-020-0556-y. BMC Neurosci. 2020. PMID: 32066381 Free PMC article.
-
Targeting NAD Metabolism for the Therapy of Age-Related Neurodegenerative Diseases.Neurosci Bull. 2024 Feb;40(2):218-240. doi: 10.1007/s12264-023-01072-3. Epub 2023 May 31. Neurosci Bull. 2024. PMID: 37253984 Free PMC article. Review.
-
Uncharacterized RNAs in Plasma of Alzheimer's Patients Are Associated with Cognitive Impairment and Show a Potential Diagnostic Power.Int J Mol Sci. 2020 Oct 15;21(20):7644. doi: 10.3390/ijms21207644. Int J Mol Sci. 2020. PMID: 33076555 Free PMC article.
-
The effect of insomnia on development of Alzheimer's disease.J Neuroinflammation. 2020 Oct 6;17(1):289. doi: 10.1186/s12974-020-01960-9. J Neuroinflammation. 2020. PMID: 33023629 Free PMC article. Review.
-
A Role for Cellular Prion Protein in Late-Onset Alzheimer's Disease: Evidence from Preclinical Studies.J Neurosci. 2018 Feb 28;38(9):2146-2148. doi: 10.1523/JNEUROSCI.3307-17.2018. J Neurosci. 2018. PMID: 29491138 Free PMC article. Review. No abstract available.
References
-
- Bondareff W, Mountjoy CQ, Roth M, Rossor MN, Iversen LL, Reynolds GP, et al. . Neuronal degeneration in locus ceruleus and cortical correlates of Alzheimer disease . Alzheimer Dis Assoc Disord 1987. ; 1 : 256 – 62 . - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

