Migraine photophobia originating in cone-driven retinal pathways
- PMID: 27190022
- PMCID: PMC4939697
- DOI: 10.1093/brain/aww119
Migraine photophobia originating in cone-driven retinal pathways
Abstract
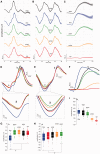
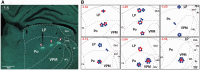
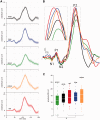
Migraine headache is uniquely exacerbated by light. Using psychophysical assessments in patients with normal eyesight we found that green light exacerbates migraine headache significantly less than white, blue, amber or red lights. To delineate mechanisms, we used electroretinography and visual evoked potential recording in patients, and multi-unit recording of dura- and light-sensitive thalamic neurons in rats to show that green activates cone-driven retinal pathways to a lesser extent than white, blue and red; that thalamic neurons are most responsive to blue and least responsive to green; and that cortical responses to green are significantly smaller than those generated by blue, amber and red lights. These findings suggest that patients' experience with colour and migraine photophobia could originate in cone-driven retinal pathways, fine-tuned in relay thalamic neurons outside the main visual pathway, and preserved by the cortex. Additionally, the findings provide substrate for the soothing effects of green light.
Keywords: electroretinography; headache; pain; thalamus; visual evoked potential.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



Comment in
-
Pupil area and photopigment spectral sensitivity are relevant to study of migraine photophobia.Brain. 2017 Jan;140(1):e2. doi: 10.1093/brain/aww274. Epub 2016 Nov 5. Brain. 2017. PMID: 27818382 Free PMC article. No abstract available.
-
Reply: Pupil area and photopigment spectral sensitivity are relevant to study of migraine photophobia.Brain. 2017 Jan;140(1):e3. doi: 10.1093/brain/aww275. Epub 2016 Nov 15. Brain. 2017. PMID: 28031225 Free PMC article. No abstract available.
Similar articles
-
Current understanding of photophobia, visual networks and headaches.Cephalalgia. 2019 Nov;39(13):1623-1634. doi: 10.1177/0333102418784750. Epub 2018 Jun 25. Cephalalgia. 2019. PMID: 29940781 Free PMC article. Review.
-
Neurobiology of Photophobia.J Neuroophthalmol. 2019 Mar;39(1):94-102. doi: 10.1097/WNO.0000000000000766. J Neuroophthalmol. 2019. PMID: 30762717 Free PMC article. Review.
-
Lack of a direct link between macular cones function and photophobia in interictal migraine.Cephalalgia. 2024 Sep;44(9):3331024241276501. doi: 10.1177/03331024241276501. Cephalalgia. 2024. PMID: 39279320
-
The migraine eye: distinct rod-driven retinal pathways' response to dim light challenges the visual cortex hyperexcitability theory.Pain. 2019 Mar;160(3):569-578. doi: 10.1097/j.pain.0000000000001434. Pain. 2019. PMID: 30376534 Free PMC article.
-
Reflexive Eye Closure in Response to Cone and Melanopsin Stimulation: A Study of Implicit Measures of Light Sensitivity in Migraine.Neurology. 2021 Oct 26;97(17):e1672-e1680. doi: 10.1212/WNL.0000000000012734. Epub 2021 Sep 7. Neurology. 2021. PMID: 34493620 Free PMC article.
Cited by
-
Evaluation of green light exposure on headache frequency and quality of life in migraine patients: A preliminary one-way cross-over clinical trial.Cephalalgia. 2021 Feb;41(2):135-147. doi: 10.1177/0333102420956711. Epub 2020 Sep 9. Cephalalgia. 2021. PMID: 32903062 Free PMC article. Clinical Trial.
-
Proteomics profiling reveals mitochondrial damage in the thalamus in a mouse model of chronic migraine.J Headache Pain. 2023 Sep 5;24(1):122. doi: 10.1186/s10194-023-01646-6. J Headache Pain. 2023. PMID: 37667199 Free PMC article.
-
Current understanding of photophobia, visual networks and headaches.Cephalalgia. 2019 Nov;39(13):1623-1634. doi: 10.1177/0333102418784750. Epub 2018 Jun 25. Cephalalgia. 2019. PMID: 29940781 Free PMC article. Review.
-
Neurobiology of Photophobia.J Neuroophthalmol. 2019 Mar;39(1):94-102. doi: 10.1097/WNO.0000000000000766. J Neuroophthalmol. 2019. PMID: 30762717 Free PMC article. Review.
-
Assessing Orofacial Pain Behaviors in Animal Models: A Review.Brain Sci. 2023 Feb 24;13(3):390. doi: 10.3390/brainsci13030390. Brain Sci. 2023. PMID: 36979200 Free PMC article. Review.
References
-
- Adams WH, Digre KB, Patel BC, Anderson RL, Warner JE, Katz BJ . The evaluation of light sensitivity in benign essential blepharospasm . Am J Ophthalmol 2006. ; 142 : 82 – 7 . - PubMed
-
- American Encephalographic Society: Guideline for standard electrode position nomenclature . J Clin Neurophysiol 1994. ; 11 ( Guideline 13 ): 111 – 3 . - PubMed
-
- Aurora SK, Cao Y, Bowyer SM, Welch KM . The occipital cortex is hyperexcitable in migraine: experimental evidence . Headache 1999. ; 39 : 469 – 76 . - PubMed
-
- Berson DM, Dunn FA, Takao M . Phototransduction by retinal ganglion cells that set the circadian clock . Science 2002. ; 295 : 1070 – 3 . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

