The unfolded protein response: mechanisms and therapy of neurodegeneration
- PMID: 27190028
- PMCID: PMC4958893
- DOI: 10.1093/brain/aww101
The unfolded protein response: mechanisms and therapy of neurodegeneration
Abstract
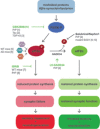
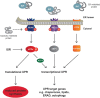
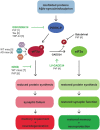
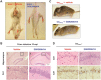
Activation of the unfolded protein response is emerging as a common theme in protein-misfolding neurodegenerative diseases, with relevant markers observed in patient tissue and mouse models. Genetic and pharmacological manipulation of the pathway in several mouse models has shown that this is not a passive consequence of the neurodegeneration process. Rather, overactivation of the protein kinase RNA-like ER kinase (PERK, encoded by EIF2AK3) branch of the unfolded protein response directly contributes to disease pathogenesis through the critical reduction in neuronal protein synthesis rates, essential for learning and memory and for neuronal survival. The pharmacological inhibition of this process in these models is strikingly neuroprotective, resulting in the discovery of the first small molecule preventing neurodegeneration and clinical disease in vivo This now represents a potential generic approach for boosting memory and preventing neurodegeneration across the spectrum of these disorders, albeit with some exceptions, independent of disease-specific proteins. Targeting the unfolded protein response, and particularly PERK-branch mediated translational failure is thus an increasingly compelling strategy for new treatments for dementia and neurodegenerative disease.
Keywords: mouse models of neurodegeneration; neurodegeneration; neuroprotection; treatment of dementia; unfolded protein response.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures
Similar articles
-
The UPR and synaptic dysfunction in neurodegeneration.Brain Res. 2016 Oct 1;1648(Pt B):530-537. doi: 10.1016/j.brainres.2016.03.029. Epub 2016 Mar 26. Brain Res. 2016. PMID: 27021956 Review.
-
PERK inhibition prevents tau-mediated neurodegeneration in a mouse model of frontotemporal dementia.Acta Neuropathol. 2015 Nov;130(5):633-42. doi: 10.1007/s00401-015-1487-z. Epub 2015 Oct 8. Acta Neuropathol. 2015. PMID: 26450683 Free PMC article.
-
Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice.Sci Transl Med. 2013 Oct 9;5(206):206ra138. doi: 10.1126/scitranslmed.3006767. Sci Transl Med. 2013. PMID: 24107777
-
The unfolded protein response in neurodegenerative disorders - therapeutic modulation of the PERK pathway.FEBS J. 2019 Jan;286(2):342-355. doi: 10.1111/febs.14422. Epub 2018 Mar 11. FEBS J. 2019. PMID: 29476642 Review.
-
Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice.Brain. 2017 Jun 1;140(6):1768-1783. doi: 10.1093/brain/awx074. Brain. 2017. PMID: 28430857 Free PMC article.
Cited by
-
CDNF Protein Therapy in Parkinson's Disease.Cell Transplant. 2019 Apr;28(4):349-366. doi: 10.1177/0963689719840290. Epub 2019 Apr 4. Cell Transplant. 2019. PMID: 30947516 Free PMC article.
-
RAN translation at C9orf72-associated repeat expansions is selectively enhanced by the integrated stress response.Nat Commun. 2017 Dec 8;8(1):2005. doi: 10.1038/s41467-017-02200-0. Nat Commun. 2017. PMID: 29222490 Free PMC article.
-
Enforced dimerization between XBP1s and ATF6f enhances the protective effects of the UPR in models of neurodegeneration.Mol Ther. 2021 May 5;29(5):1862-1882. doi: 10.1016/j.ymthe.2021.01.033. Epub 2021 Feb 3. Mol Ther. 2021. PMID: 33545358 Free PMC article.
-
Increased expression of ER stress, inflammasome activation, and mitochondrial biogenesis-related genes in peripheral blood mononuclear cells in major depressive disorder.Mol Psychiatry. 2025 Feb;30(2):574-586. doi: 10.1038/s41380-024-02695-2. Epub 2024 Aug 22. Mol Psychiatry. 2025. PMID: 39174649 Free PMC article.
-
Understanding the Unfolded Protein Response (UPR) Pathway: Insights into Neuropsychiatric Disorders and Therapeutic Potentials.Biomol Ther (Seoul). 2024 Mar 1;32(2):183-191. doi: 10.4062/biomolther.2023.181. Biomol Ther (Seoul). 2024. PMID: 38410073 Free PMC article. Review.
References
-
- Atkin JD, Farg MA, Walker AK, McLean C, Tomas D, Horne MK. Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neurobiol Dis 2008; 30: 400–7. - PubMed
-
- Burté F, Carelli V, Chinnery PF, Yu-Wai-Man P. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat Rev Neurol 2015; 11: 11–24. - PubMed
-
- Chen A, Muzzio IA, Malleret G, Bartsch D, Verbitsky M, Pavlidis P, et al. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron 2003; 39: 655–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

