Trimethylamine and Trimethylamine N-Oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-Mediated Host-Microbiome Metabolic Axis Implicated in Health and Disease
- PMID: 27190056
- PMCID: PMC5074467
- DOI: 10.1124/dmd.116.070615
Trimethylamine and Trimethylamine N-Oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-Mediated Host-Microbiome Metabolic Axis Implicated in Health and Disease
Erratum in
-
Correction to "Trimethylamine and Trimethylamine N-Oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-Mediated Host-Microbiome Metabolic Axis Implicated in Health and Disease".Drug Metab Dispos. 2016 Dec;44(12):1949. doi: 10.1124/dmd.115.070615err. Drug Metab Dispos. 2016. PMID: 27806989 Free PMC article. No abstract available.
Abstract
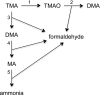
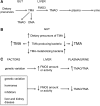
Flavin-containing monooxygenase 3 (FMO3) is known primarily as an enzyme involved in the metabolism of therapeutic drugs. On a daily basis, however, we are exposed to one of the most abundant substrates of the enzyme trimethylamine (TMA), which is released from various dietary components by the action of gut bacteria. FMO3 converts the odorous TMA to nonodorous TMA N-oxide (TMAO), which is excreted in urine. Impaired FMO3 activity gives rise to the inherited disorder primary trimethylaminuria (TMAU). Affected individuals cannot produce TMAO and, consequently, excrete large amounts of TMA. A dysbiosis in gut bacteria can give rise to secondary TMAU. Recently, there has been much interest in FMO3 and its catalytic product, TMAO, because TMAO has been implicated in various conditions affecting health, including cardiovascular disease, reverse cholesterol transport, and glucose and lipid homeostasis. In this review, we consider the dietary components that can give rise to TMA, the gut bacteria involved in the production of TMA from dietary precursors, the metabolic reactions by which bacteria produce and use TMA, and the enzymes that catalyze the reactions. Also included is information on bacteria that produce TMA in the oral cavity and vagina, two key microbiome niches that can influence health. Finally, we discuss the importance of the TMA/TMAO microbiome-host axis in health and disease, considering factors that affect bacterial production and host metabolism of TMA, the involvement of TMAO and FMO3 in disease, and the implications of the host-microbiome axis for management of TMAU.
Copyright © 2016 The Author(s).
Figures

References
-
- Al-Waiz M, Ayesh R, Mitchell SC, Idle JR, Smith RL. (1987c) A genetic polymorphism of the N-oxidation of trimethylamine in humans. Clin Pharmacol Ther 42:588–594. - PubMed
-
- Al-Waiz M, Ayesh R, Mitchell SC, Idle JR, Smith RL. (1989) Trimethylaminuria: the detection of carriers using a trimethylamine load test. J Inherit Metab Dis 12:80–85. - PubMed
-
- Al-Waiz M, Mikov M, Mitchell SC, Smith RL. (1992) The exogenous origin of trimethylamine in the mouse. Metabolism 41:135–136. - PubMed
-
- Al-Waiz M, Mitchell SC, Idle JR, Smith RL. (1987a) The metabolism of 14C-labelled trimethylamine and its N-oxide in man. Xenobiotica 17:551–558. - PubMed
-
- Al-Waiz M, Mitchell SC, Idle JR, Smith RL. (1987b) The relative importance of N-oxidation and N-demethylation in the metabolism of trimethylamine in man. Toxicology 43:117–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

