β2-Adrenergic agonists attenuate organic dust-induced lung inflammation
- PMID: 27190062
- PMCID: PMC4967192
- DOI: 10.1152/ajplung.00125.2016
β2-Adrenergic agonists attenuate organic dust-induced lung inflammation
Abstract
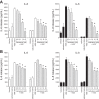
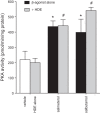
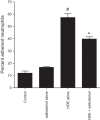
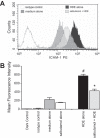
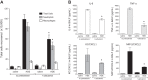
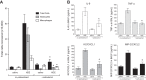
Agricultural dust exposure results in significant lung inflammation, and individuals working in concentrated animal feeding operations (CAFOs) are at risk for chronic airway inflammatory diseases. Exposure of bronchial epithelial cells to aqueous extracts of hog CAFO dusts (HDE) leads to inflammatory cytokine production that is driven by protein kinase C (PKC) activation. cAMP-dependent protein kinase (PKA)-activating agents can inhibit PKC activation in epithelial cells, leading to reduced inflammatory cytokine production following HDE exposure. β2-Adrenergic receptor agonists (β2-agonists) activate PKA, and we hypothesized that β2-agonists would beneficially impact HDE-induced adverse airway inflammatory consequences. Bronchial epithelial cells were cultured with the short-acting β2-agonist salbutamol or the long-acting β2-agonist salmeterol prior to stimulation with HDE. β2-Agonist treatment significantly increased PKA activation and significantly decreased HDE-stimulated IL-6 and IL-8 production in a concentration- and time-dependent manner. Salbutamol treatment significantly reduced HDE-induced intracellular adhesion molecule-1 expression and neutrophil adhesion to epithelial cells. Using an established intranasal inhalation exposure model, we found that salbutamol pretreatment reduced airway neutrophil influx and IL-6, TNF-α, CXCL1, and CXCL2 release in bronchoalveolar lavage fluid following a one-time exposure to HDE. Likewise, when mice were pretreated daily with salbutamol prior to HDE exposure for 3 wk, HDE-induced neutrophil influx and inflammatory mediator production were also reduced. The severity of HDE-induced lung pathology in mice repetitively exposed to HDE for 3 wk was also decreased with daily salbutamol pretreatment. Together, these results support the need for future clinical investigations to evaluate the utility of β2-agonist therapies in the treatment of airway inflammation associated with CAFO dust exposure.
Keywords: agricultural dusts; bronchial epithelial cells; concentrated animal feeding operations; lung inflammation; β2-agonists.
Figures



Similar articles
-
Proteases in agricultural dust induce lung inflammation through PAR-1 and PAR-2 activation.Am J Physiol Lung Cell Mol Physiol. 2015 Aug 15;309(4):L388-99. doi: 10.1152/ajplung.00025.2015. Epub 2015 Jun 19. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26092994 Free PMC article.
-
Alcohol exposure alters mouse lung inflammation in response to inhaled dust.Nutrients. 2012 Jul;4(7):695-710. doi: 10.3390/nu4070695. Epub 2012 Jul 4. Nutrients. 2012. PMID: 22852058 Free PMC article.
-
cAMP-dependent protein kinase activation decreases cytokine release in bronchial epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2014 Oct 15;307(8):L643-51. doi: 10.1152/ajplung.00373.2013. Epub 2014 Aug 22. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 25150062 Free PMC article.
-
Anabolic and lipolytic actions of beta2 -agonists in humans and antidoping challenges.Drug Test Anal. 2020 May;12(5):597-609. doi: 10.1002/dta.2728. Epub 2020 Feb 20. Drug Test Anal. 2020. PMID: 31960603 Review.
-
Looking for agonists of β2 adrenergic receptor from Fuzi and Chuanwu by virtual screening and dual-luciferase reporter assay.J Asian Nat Prod Res. 2016 Jun;18(6):550-61. doi: 10.1080/10286020.2015.1123692. Epub 2015 Dec 24. J Asian Nat Prod Res. 2016. PMID: 26700061 Review.
Cited by
-
N-Desmethylclozapine, Fluoxetine, and Salmeterol Inhibit Postentry Stages of the Dengue Virus Life Cycle.Antimicrob Agents Chemother. 2016 Oct 21;60(11):6709-6718. doi: 10.1128/AAC.01367-16. Print 2016 Nov. Antimicrob Agents Chemother. 2016. PMID: 27572397 Free PMC article.
-
Isoprenaline protects intestinal stem cells from chemotherapy-induced damage.Br J Pharmacol. 2020 Feb;177(3):687-700. doi: 10.1111/bph.14883. Epub 2020 Jan 3. Br J Pharmacol. 2020. PMID: 31648381 Free PMC article.
-
Dimethylarginine dimethylaminohydrolase (DDAH) overexpression enhances wound repair in airway epithelial cells exposed to agricultural organic dust.Inhal Toxicol. 2018 Feb;30(3):133-139. doi: 10.1080/08958378.2018.1474976. Epub 2018 May 25. Inhal Toxicol. 2018. PMID: 29793367 Free PMC article.
-
Lipids potentially contribute to exacerbated inflammatory markers in Metabolic Syndrome mice acutely following pulmonary nanoparticle exposure.J Toxicol Environ Health A. 2025 Jul 2:1-19. doi: 10.1080/15287394.2025.2527646. Online ahead of print. J Toxicol Environ Health A. 2025. PMID: 40601432
-
Systemic Sympathoexcitation Was Associated with Paraventricular Hypothalamic Phosphorylation of Synaptic CaMKIIα and MAPK/ErK.Front Neurosci. 2017 Aug 3;11:447. doi: 10.3389/fnins.2017.00447. eCollection 2017. Front Neurosci. 2017. PMID: 28824368 Free PMC article.
References
-
- Bloemen PG, van den Tweel MC, Henricks PA, Engels F, Wagenaar SS, Rutten AA, Nijkamp FP. Expression and modulation of adhesion molecules on human bronchial epithelial cells. Am J Respir Cell Mol Biol 9: 586–593, 1993. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

