Early coagulation events induce acute lung injury in a rat model of blunt traumatic brain injury
- PMID: 27190065
- PMCID: PMC4967191
- DOI: 10.1152/ajplung.00429.2015
Early coagulation events induce acute lung injury in a rat model of blunt traumatic brain injury
Abstract
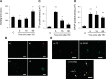
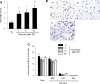
Acute lung injury (ALI) and systemic coagulopathy are serious complications of traumatic brain injury (TBI) that frequently lead to poor clinical outcomes. Although the release of tissue factor (TF), a potent initiator of the extrinsic pathway of coagulation, from the injured brain is thought to play a key role in coagulopathy after TBI, its function in ALI following TBI remains unclear. In this study, we investigated whether the systemic appearance of TF correlated with the ensuing coagulopathy that follows TBI in ALI using an anesthetized rat blunt trauma TBI model. Blood and lung samples were obtained after TBI. Compared with controls, pulmonary edema and increased pulmonary permeability were observed as early as 5 min after TBI without evidence of norepinephrine involvement. Systemic TF increased at 5 min and then diminished 60 min after TBI. Lung injury and alveolar hemorrhaging were also observed as early as 5 min after TBI. A biphasic elevation of TF was observed in the lungs after TBI, and TF-positive microparticles (MPs) were detected in the alveolar spaces. Fibrin(ogen) deposition was also observed in the lungs within 60 min after TBI. Additionally, preadministration of a direct thrombin inhibitor, Refludan, attenuated lung injuries, thus implicating thrombin as a direct participant in ALI after TBI. The results from this study demonstrated that enhanced systemic TF may be an initiator of coagulation activation that contributes to ALI after TBI.
Keywords: acute lung injury; coagulation pathway; coagulopathy; tissue factor; traumatic brain injury.
Copyright © 2016 the American Physiological Society.
Figures









References
-
- Carlson RW, Schaeffer RC Jr, Michaels SG, Weil MH. Pulmonary edema following intracranial hemorrhage. Chest 75: 731–734, 1979. - PubMed
-
- Castellino FJ, Chapman MP, Donahue DL, Thomas S, Moore EE, Wohlauer MV, Fritz B, Yount R, Ploplis V, Davis P, Evans E, Walsh M. Traumatic brain injury causes platelet adenosine diphosphate and arachidonic acid receptor inhibition independent of hemorrhagic shock in humans and rats. J Trauma Acute Care Surg 76: 1169–1176, 2014. - PMC - PubMed
-
- Chen HI, Sun SC, Chai CY. Pulmonary edema and hemorrhage resulting from cerebral compression. Am J Physiol 224: 223–229, 1973. - PubMed
-
- Cohen MJ, Brohi K, Ganter MT, Manley GT, Mackersie RC, Pittet JF. Early coagulopathy after traumatic brain injury: the role of hypoperfusion and the protein C pathway. J Trauma 63: 1254–1261; discussion 1261–1252, 2007. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

