VAR2CSA Domain-Specific Analysis of Naturally Acquired Functional Antibodies to Plasmodium falciparum Placental Malaria
- PMID: 27190180
- PMCID: PMC4957442
- DOI: 10.1093/infdis/jiw197
VAR2CSA Domain-Specific Analysis of Naturally Acquired Functional Antibodies to Plasmodium falciparum Placental Malaria
Abstract
Background: Placental malaria is caused by Plasmodium falciparum-infected erythrocytes (IEs) that surface-express VAR2CSA and bind chondroitin sulfate A. The inflammatory response to placenta-sequestered parasites is associated with poor pregnancy outcomes, and protection may be mediated in part by VAR2CSA antibodies that block placental IE adhesion.
Methods: In this study, we used a new approach to assess VAR2CSA domains for functional epitopes recognized by naturally acquired antibodies. Antigen-specific immunoglobulin (Ig) G targeting Duffy binding-like (DBL) domains from different alleles were sequentially purified from plasma pooled from multigravid women and then characterized using enzyme-linked immunosorbent assay, flow cytometry, and antiadhesion assays.
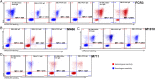
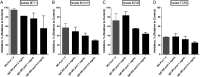
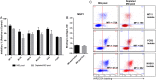
Results: Different DBL domain-specific IgGs could react to homologous as well as heterologous antigens and parasites, suggesting that conserved epitopes are shared between allelic variants. Homologous blocking of IE binding was observed with ID1-DBL2-ID2a-, DBL4-, and DBL5-specific IgG (range, 42%-75%), whereas partial cross-inhibition activity was observed with purified IgG specific to ID1-DBL2-ID2a and DBL4 antigens. Plasma retained broadly neutralizing activity after complete depletion of these VAR2CSA specificities.
Conclusions: Broadly neutralizing antibodies of multigravidae are not depleted on VAR2CSA recombinant antigens, and hence development of VAR2CSA vaccines based on a single construct and variant might induce antibodies with limited broadly neutralizing activity.
Keywords: DBL domains; VAR2CSA; functional antibody; malaria; multigravidae; pregnancy; vaccine.
Published by Oxford University Press for the Infectious Diseases Society of America 2016. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures

 ), M711 (
), M711 ( ), and M1010 (
), and M1010 ( ). Boundaries of each construct are indicated relative to FCR3 VAR2CSA (GenBank accession No. KU665624). The same residue limit was used for all DBL4ε and DBL5ε constructs [25]. Different boundaries were used for ID1-DBL2X-ID2a-1010 and ID1-DBL2X-ID2a-711 (GenBank for accession No. KU665625), and ID1-DBL2X-ID2a-FCR3 (GenBank for accession No. KU665626) recombinant constructs. B, The MG pool was screened by means of enzyme-linked immunosorbent assay (ELISA) against the VAR2CSA DBL domain recombinant and AMA1 (used as non-VAR2CSA antigen control) antigens. Data are presented as level of antibody binding to the recombinant proteins, measured by optical density (OD). Bars represent means and standard deviations of duplicate wells. Abbreviations: ATS, acidic terminal sequence; TM, transmembrane.
). Boundaries of each construct are indicated relative to FCR3 VAR2CSA (GenBank accession No. KU665624). The same residue limit was used for all DBL4ε and DBL5ε constructs [25]. Different boundaries were used for ID1-DBL2X-ID2a-1010 and ID1-DBL2X-ID2a-711 (GenBank for accession No. KU665625), and ID1-DBL2X-ID2a-FCR3 (GenBank for accession No. KU665626) recombinant constructs. B, The MG pool was screened by means of enzyme-linked immunosorbent assay (ELISA) against the VAR2CSA DBL domain recombinant and AMA1 (used as non-VAR2CSA antigen control) antigens. Data are presented as level of antibody binding to the recombinant proteins, measured by optical density (OD). Bars represent means and standard deviations of duplicate wells. Abbreviations: ATS, acidic terminal sequence; TM, transmembrane.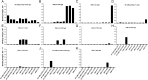

References
-
- Muthusamy A, Achur RN, Bhavanandan VP, Fouda GG, Taylor DW, Gowda DC. Plasmodium falciparum-infected erythrocytes adhere both in the intervillous space and on the villous surface of human placenta by binding to the low-sulfated chondroitin sulfate proteoglycan receptor. Am J Pathol 2004; 164:2013–25. - PMC - PubMed
-
- Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg 2003; 68:115–9. - PubMed
-
- Shulman CE, Graham WJ, Jilo H et al. . Malaria is an important cause of anaemia in primigravidae: evidence from a district hospital in coastal Kenya. Trans R Soc Trop Med Hyg 1996; 90:535–9. - PubMed
-
- Steketee RW, Wirima JJ, Hightower AW, Slutsker L, Heymann DL, Breman JG. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. Am J Trop Med Hyg 1996; 55(1 Suppl):33–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

