Hepatitis B Virus Reactivation in the Setting of Cancer Chemotherapy and Other Immunosuppressive Drug Therapy
- PMID: 27190320
- PMCID: PMC4889897
- DOI: 10.1093/cid/ciw043
Hepatitis B Virus Reactivation in the Setting of Cancer Chemotherapy and Other Immunosuppressive Drug Therapy
Abstract
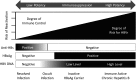
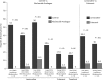
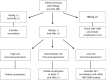
Hepatitis B virus reactivation (HBVr) is an important complication of immunosuppressive drug therapy (ISDT). It can occur with active or resolved hepatitis B virus (HBV) infection with a clinical spectrum that ranges from mild elevations in liver tests to fulminant hepatic failure. The risk of it occurring is determined by the interplay between HBV serological status, level of viremia, and the immunosuppressive potency of the drug(s) used. Reactivation is most common during treatment of hematologic malignancies but also occurs with chemotherapy for breast cancer and numerous other solid organ malignancies, organ transplant, and immune suppression for nonmalignant conditions. The expansion of new biologic treatments for malignant and nonmalignant disorders has enlarged the population at risk. Increased awareness of HBVr among healthcare providers who prescribe ISDT, adoption of routine HBV screening, and linking the results of screening to antiviral prophylaxis are needed to reduce the incidence of this potentially fatal but preventable disorder.
Keywords: HBVr; ISDT; cancer; chemotherapy; liver.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures
References
-
- Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015; 148:221–44. - PubMed
-
- Gonzalez SA, Perrillo RP. Reactivation of hepatitis B virus due to chemotherapy or immunosuppressive drug therapy. In: Liaw YF, Zoulim F. Hepatitis B virus in human diseases. 1st ed New York: Springer, 2015.
-
- Hui CK, Cheung WW, Zhang HY et al. . Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology 2006; 131:59–68. - PubMed
-
- Perrillo RP. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology 2001; 120:1009–22. - PubMed
-
- Lau GK, Yiu HH, Fong DY et al. . Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology 2003; 125:1742–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

