Outcomes in patients with chronic kidney disease not on dialysis receiving extended dosing regimens of darbepoetin alfa: long-term results of the EXTEND observational cohort study
- PMID: 27190334
- PMCID: PMC5146706
- DOI: 10.1093/ndt/gfw047
Outcomes in patients with chronic kidney disease not on dialysis receiving extended dosing regimens of darbepoetin alfa: long-term results of the EXTEND observational cohort study
Abstract
Background: Extended dosing of the erythropoiesis-stimulating agent (ESA) darbepoetin alfa (DA) once biweekly or monthly reduces anaemia treatment burden. This observational study assessed outcomes and dosing patterns in patients with chronic kidney disease not on dialysis (CKD-NoD) commencing extended dosing of DA.
Methods: Adult CKD-NoD patients starting extended dosing of DA in Europe or Australia in June 2006 or later were followed up until December 2012. Outcomes included haemoglobin (Hb) concentration, ESA dosing, mortality rates and receipt of dialysis and renal transplantation. Subgroup analyses were conducted for selected outcomes.
Results: Of 6035 enrolled subjects, 5723 (94.8%) met analysis criteria; 1795 (29.7%) received dialysis and 238 (3.9%) underwent renal transplantation. Mean (standard deviation) Hb concentration at commencement of extended dosing was 11.0 (1.5) g/dL. Mean [95% confidence interval (CI)] Hb 12 months after commencement of extended dosing (primary outcome) was 11.6 g/dL (11.5, 11.6) overall and was similar across countries, with no differences between subjects previously treated with an ESA versus ESA-naïve subjects, subjects with versus without prior renal transplant or diabetics versus non-diabetics. Weekly ESA dose gradually decreased following commencement of extended DA dosing and was similar across subgroups. The decrease in weekly DA dose was accompanied by an increase in the proportion of patients receiving iron therapy. Hb concentrations declined following changes in ESA labels and treatment guidelines. The mortality rate (95% CI) was 7.06 (6.68, 7.46) deaths per 100 years of follow-up. Subjects alive at study end had stable Hb concentrations in the preceding year, while those who died had lower and declining Hb concentrations in their last year.
Conclusions: Long-term, extended dosing of DA maintained Hb concentrations in patients already treated with an ESA and corrected and maintained Hb in ESA-naïve patients.
Keywords: anaemia; chronic kidney disease; darbepoetin alfa; erythropoiesis-stimulating agent; extended dosing.
© The Author 2016. Published by Oxford University Press on behalf of ERA-EDTA.
Figures
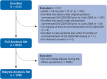



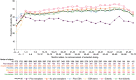
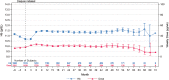
References
-
- Macdougall IC. Optimizing the use of erythropoietic agents—pharmacokinetic and pharmacodynamic considerations. Nephrol Dial Transplant 2002; 17 (Suppl 5): 66–70 - PubMed
-
- Cody J, Daly C, Campbell M et al. . Recombinant human erythropoietin for chronic renal failure anaemia in pre-dialysis patients. Cochrane Database Syst Rev 2005: CD003266. - PubMed
-
- Singh AK, Szczech L, Tang KL et al. . Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 - PubMed
-
- Drueke TB, Locatelli F, Clyne N et al. . Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355: 2071–2084 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

