15-Deoxy-Δ(12,14)-prostaglandin J2 Induces Apoptosis and Upregulates SOCS3 in Human Thyroid Cancer Cells
- PMID: 27190500
- PMCID: PMC4852108
- DOI: 10.1155/2016/4106297
15-Deoxy-Δ(12,14)-prostaglandin J2 Induces Apoptosis and Upregulates SOCS3 in Human Thyroid Cancer Cells
Abstract
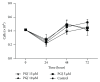
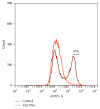
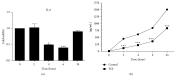
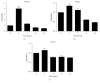
The cyclopentenone prostaglandin 15-deoxy-Δ(12,14)-prostaglandin J2 (15d-PGJ2) is a natural ligand of peroxisome proliferator-activated receptor gamma (PPAR-γ) and a potential mediator of apoptosis in cancer cells. In the present study, we evaluated the effect of 15d-PGJ2 in human thyroid papillary carcinoma cells (TPC-1) using different doses of 15d-PGJ2 (0.6 to 20 μM) to determine IC50 (9.3 μM) via the MTT assay. The supernatant culture medium of the TPC-1 cells that was treated either with 15d-PGJ2 or with vehicle (control) for 24 hours was assessed for IL-6 secretion via CBA assay. RT-qPCR was used to evaluate mRNA expression of IL-6, SOCS1, SOCS3, and STAT3. TPC-1 cells treated with 15d-PGJ2 decreased the secretion and expression of IL-6 and STAT3, while it increased SOCS1 and SOCS3. Overall, we demonstrated that 15d-PGJ2 downregulated IL-6 signaling pathway and led TPC-1 cells into apoptosis. In conclusion, 15d-PGJ2 shows the potential to become a new therapeutic approach for thyroid tumors.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

