Chinese Herbal Medicine as Adjunctive Therapy to Chemotherapy for Breast Cancer: A Systematic Review and Meta-Analysis
- PMID: 27190531
- PMCID: PMC4842043
- DOI: 10.1155/2016/3281968
Chinese Herbal Medicine as Adjunctive Therapy to Chemotherapy for Breast Cancer: A Systematic Review and Meta-Analysis
Abstract
Chinese herbal medicine (CHM) has been increasingly employed during therapy for breast cancer, but its efficacy remains a matter of debate. This systematic review examined randomized controlled trials to provide a critical evaluation of this treatment. The results demonstrated that the combined use of CHM with chemotherapy may improve the immediate tumor response and reduce chemotherapy-associated adverse events. Our findings highlight the poor quality of Chinese studies, and additional well-designed randomized controlled trials addressing the role of CHM are warranted. The lack of molecular-based evidence for CHM and Zheng has resulted in a limited understanding and acceptance of CHM and traditional Chinese medicine in Western countries. We believe that researchers should immediately explore a CHM-based cure, and CHM should be applied to routine care as soon as conclusive data are available.
Figures
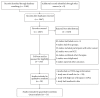





























References
-
- Del Mastro L., Fabi A., Mansutti M., et al. Randomised phase 3 open-label trial of first-line treatment with gemcitabine in association with docetaxel or paclitaxel in women with metastatic breast cancer: a comparison of different schedules and treatments. BMC Cancer. 2013;13, article 164 doi: 10.1186/1471-2407-13-164. - DOI - PMC - PubMed
-
- Amadori D., Nanni O., Marangolo M., et al. Disease-free survival advantage of adjuvant cyclophosphamide, methotrexate, and fluorouracil in patients with node-negative, rapidly proliferating breast cancer: a randomized multicenter study. Journal of Clinical Oncology. 2000;18(17):3125–3134. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

