Poly(lactic-co-glycolic) Acid-Chitosan Dual Loaded Nanoparticles for Antiretroviral Nanoformulations
- PMID: 27190651
- PMCID: PMC4852115
- DOI: 10.1155/2016/3810175
Poly(lactic-co-glycolic) Acid-Chitosan Dual Loaded Nanoparticles for Antiretroviral Nanoformulations
Abstract
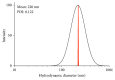
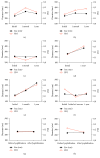
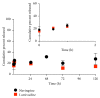
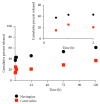
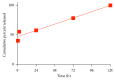
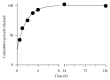
Poly(lactic-co-glycolic acid) (PLGA) chitosan (CS) coated nanoparticles (NPs) were loaded with two antiretrovirals (ARVs) either lamivudine (LMV) which is hydrophilic or nevirapine (NVP) which is hydrophobic or both LMV and NVP. These ARVs are of importance in resource-limited settings, where they are commonly used in human immunodeficiency virus (HIV-1) treatment due to affordability and accessibility. NPs prepared by a water-oil-water emulsion and reduced pressure solvent evaporation technique were determined to have a positive zeta potential, a capsule-like morphology, and an average hydrodynamic diameter of 240 nm. Entrapment of NVP as a single ARV had a notable increase in NP size compared to LMV alone or in combination with LMV. NPs stored at room temperature in distilled water maintained size, polydispersity (PDI), and zeta potential for one year. No changes in size, PDI, and zeta potential were observed for NPs in 10% sucrose in lyophilized or nonlyophilized states stored at 4°C and -20°C, respectively. Freezing NPs in the absence of sucrose increased NP size. Drug loading, encapsulation efficiency, and kinetic release profiles were quantified by high performance liquid chromatography (HPLC). Our novel nanoformulations have the potential to improve patient outcomes and expand drug access in resource-limited countries for the treatment of HIV-1.
Figures

References
-
- World Health Organization. HIV/AIDS Fact Sheet. http://www.who.int/mediacentre/factsheets/fs360/en/
-
- UNAIDS. How AIDS changed everything—MDG6: 15 years, 15 lessons of hope from the AIDS response. http://www.unaids.org/en/resources/documents/2015/MDG6_15years-15lessons....
-
- World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva, Switzerland: World Health Organization; 2013. http://www.who.int/hiv/pub/guidelines/arv2013/download/en/
-
- National Institutes of Health. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatmen....
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

