Sieving characteristics of cytokine- and peroxide-induced epithelial barrier leak: Inhibition by berberine
- PMID: 27190695
- PMCID: PMC4867402
- DOI: 10.4291/wjgp.v7.i2.223
Sieving characteristics of cytokine- and peroxide-induced epithelial barrier leak: Inhibition by berberine
Abstract
Aim: To study whether the inflammatory bowel disease (IBD) colon which exhibits varying severity and cytokine levels across its mucosa create varying types of transepithelial leak.
Methods: We examined the effects of tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-1-β (IL1β) and hydrogen peroxide (H2O2) - singly and in combinations - on barrier function of CACO-2 cell layers. Our focus was on the type (not simply the magnitude) of transepithelial leak generated by these agents as measured by transepithelial electrical resistance (TER) and transepithelial flux of (14)C-D-mannitol, (3)H-Lactulose and (14)C-Polyethylene glycol as radiolabeled probe molecules. The isoquinoline alkaloid, berberine, was then examined for its ability to reduce specific types of transepithelial leak.
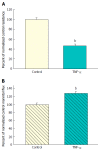
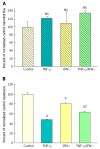
Results: Exposure to TNF-α alone (200 ng/mL; 48 h) induced a 50% decrease in TER, i.e., increased leak of Na(+) and Cl(-) - with only a marginal but statistically significant increase in transepithelial leak of (14)C-mannitol (Jm). Exposure to TNF-α + IFN-γ (200 ng/mL; 48 h) + IL1β (50 ng/mL; 48 h) did not increase the TER change (from TNF-α alone), but there was now a 100% increase in Jm. There however was no increase in transepithelial leak of two larger probe molecules, (3)H-lactulose and (14)C-polyethylene glycol (PEG). However, exposure to TNF-α + IFN-γ + IL1β followed by a 5 h exposure to 2 mmol/L H2O2 resulted in a 500% increase in (14)C-PEG leak as well as leak to the luminal mitogen, epidermal growth factor.
Conclusion: This model of graded transepithelial leak is useful in evaluating therapeutic agents reducing IBD morbidity by reducing barrier leak to various luminal substances.
Keywords: Berberine; CACO-2; Crohn’s disease; Cytokine; Intestine; Micronutrient; Tight junction; Ulcerative colitis.
Figures






References
-
- Bruewer M, Samarin S, Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci. 2006;1072:242–252. - PubMed
-
- Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G851–G857. - PubMed
-
- Shen L, Turner JR. Role of epithelial cells in initiation and propagation of intestinal inflammation. Eliminating the static: tight junction dynamics exposed. Am J Physiol Gastrointest Liver Physiol. 2006;290:G577–G582. - PubMed
-
- Thoreson R, Cullen JJ. Pathophysiology of inflammatory bowel disease: an overview. Surg Clin North Am. 2007;87:575–585. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

