BMP4 Signaling Is Able to Induce an Epithelial-Mesenchymal Transition-Like Phenotype in Barrett's Esophagus and Esophageal Adenocarcinoma through Induction of SNAIL2
- PMID: 27191723
- PMCID: PMC4871520
- DOI: 10.1371/journal.pone.0155754
BMP4 Signaling Is Able to Induce an Epithelial-Mesenchymal Transition-Like Phenotype in Barrett's Esophagus and Esophageal Adenocarcinoma through Induction of SNAIL2
Erratum in
-
Correction: BMP4 Signaling Is Able to Induce an Epithelial-Mesenchymal Transition-Like Phenotype in Barrett's Esophagus and Esophageal Adenocarcinoma through Induction of SNAIL2.PLoS One. 2016 Jun 29;11(6):e0158755. doi: 10.1371/journal.pone.0158755. eCollection 2016. PLoS One. 2016. PMID: 27355344 Free PMC article.
Abstract
Background: Bone morphogenetic protein 4 (BMP4) signaling is involved in the development of Barrett's esophagus (BE), a precursor of esophageal adenocarcinoma (EAC). In various cancers, BMP4 has been found to induce epithelial-mesenchymal transition (EMT) but its function in the development of EAC is currently unclear.
Aim: To investigate the expression of BMP4 and several members of the BMP4 pathway in EAC. Additionally, to determine the effect of BMP4 signaling in a human Barrett's esophagus (BAR-T) and adenocarcinoma (OE33) cell line.
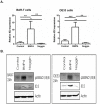
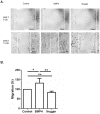
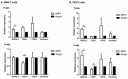
Methods: Expression of BMP4, its downstream target ID2 and members of the BMP4 pathway were determined by Q-RT-PCR, immunohistochemistry and Western blot analysis using biopsy samples from EAC patients. BAR-T and OE33 cells were incubated with BMP4 or the BMP4 antagonist, Noggin, and cell viability and migration assays were performed. In addition, expression of factors associated with EMT (SNAIL2, CDH1, CDH2 and Vimentin) was evaluated by Q-RT-PCR and Western blot analysis.
Results: Compared to squamous epithelium (SQ), BMP4 expression was significantly upregulated in EAC and BE. In addition, the expression of ID2 was significantly upregulated in EAC and BE compared to SQ. Western blot analysis confirmed our results, showing an upregulated expression of BMP4 and ID2 in both BE and EAC. In addition, more phosphorylation of SMAD1/5/8 was observed. BMP4 incubation inhibited cell viability, but induced cell migration in both BAR-T and OE33 cells. Upon BMP4 incubation, SNAIL2 expression was significantly upregulated in BAR-T and OE33 cells while CDH1 expression was significantly downregulated. These results were confirmed by Western blot analysis.
Conclusion: Our results indicate active BMP4 signaling in BE and EAC and suggest that this results in an invasive phenotype by inducing an EMT-like response through upregulation of SNAIL2 and subsequent downregulation of CDH1.
Conflict of interest statement
Figures

References
-
- Haggitt RC. Barrett's esophagus, dysplasia, and adenocarcinoma. Hum Pathol 1994;25(10):982–993. - PubMed
-
- van Baal JW, Milano F, Rygiel AM, Bergman JJ, Rosmolen WD, van Deventer SJ, et al. A comperative analysis by SAGE of gene expression profiles of Barrett’s esophagus, normal squamous esophagus, and gastric cardia. Gastroenterology 2005;129 (4):1274–1281. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

