Combined experience of six independent laboratories attempting to create an Ewing sarcoma mouse model
- PMID: 27191748
- PMCID: PMC5470957
- DOI: 10.18632/oncotarget.9388
Combined experience of six independent laboratories attempting to create an Ewing sarcoma mouse model
Abstract
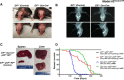
Ewing sarcoma (ES) involves a tumor-specific chromosomal translocation that produces the EWS-FLI1 protein, which is required for the growth of ES cells both in vitro and in vivo. However, an EWS-FLI1-driven transgenic mouse model is not currently available. Here, we present data from six independent laboratories seeking an alternative approach to express EWS-FLI1 in different murine tissues. We used the Runx2, Col1a2.3, Col1a3.6, Prx1, CAG, Nse, NEFL, Dermo1, P0, Sox9 and Osterix promoters to target EWS-FLI1 or Cre expression. Additional approaches included the induction of an endogenous chromosomal translocation, in utero knock-in, and the injection of Cre-expressing adenovirus to induce EWS-FLI1 expression locally in multiple lineages. Most models resulted in embryonic lethality or developmental defects. EWS-FLI1-induced apoptosis, promoter leakiness, the lack of potential cofactors, and the difficulty of expressing EWS-FLI1 in specific sites were considered the primary reasons for the failed attempts to create a transgenic mouse model of ES.
Keywords: EWS-FLI1; EWS-FLI1 driven transgenic mouse model; Ewing sarcoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Arndt CA, Crist WM. Common musculoskeletal tumors of childhood and adolescence. The New England journal of medicine. 1999;341:342–352. - PubMed
-
- Potratz J, Dirksen U, Jurgens H, Craft A. Ewing sarcoma: clinical state-of-the-art. Pediatric hematology and oncology. 2012;29:1–11. - PubMed
-
- Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, Kovar H, Joubert I, de Jong P, Rouleau G, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–165. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

