Pemetrexed/Carboplatin/Bevacizumab followed by Maintenance Pemetrexed/Bevacizumab in Hispanic Patients with Non-Squamous Non-Small Cell Lung Cancer: Outcomes according to Thymidylate Synthase Expression
- PMID: 27191954
- PMCID: PMC4871516
- DOI: 10.1371/journal.pone.0154293
Pemetrexed/Carboplatin/Bevacizumab followed by Maintenance Pemetrexed/Bevacizumab in Hispanic Patients with Non-Squamous Non-Small Cell Lung Cancer: Outcomes according to Thymidylate Synthase Expression
Abstract
Objective: To evaluate the efficacy and safety of pemetrexed, carboplatin and bevacizumab (PCB) followed by maintenance therapy with pemetrexed and bevacizumab (PB) in chemotherapy-naïve patients with stage IV non-squamous non-small cell lung cancer (NSCLC) through the influence of thymidylate synthase (TS) protein and mRNA expression on several outcomes. The primary endpoints were the overall response rate (ORR), progression-free survival (PFS) and overall survival (OS).
Methods: A cohort of 144 patients were administered pemetrexed (500 mg/m2), carboplatin (AUC, 5.0 mg/ml/min) and bevacizumab (7.5 mg/kg) intravenously every three weeks for up to four cycles. Maintenance PB was administered until disease progression or unacceptable toxicity.
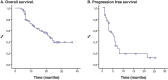
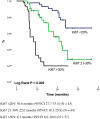
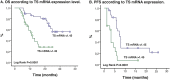
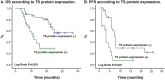
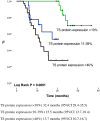
Results: One hundred forty-four Colombian patients with a median follow-up of 13.8 months and a median number of 6 maintenance cycles (range, 1-32) were assessed. The ORR among the patients was 66% (95% CI, 47% to 79%). The median PFS and (OS) rates were 7.9 months (95% CI, 5.9-10.0 months) and 21.4 months (95% CI, 18.3 to 24.4 months), respectively. We documented grade 3/4 hematologic toxicities, including anemia (14%), neutropenia (8%), and thrombocytopenia (16%). The identified grade 3/4 non-hematologic toxicities were proteinuria (2%), venous thrombosis (4%), fatigue (11%), infection (6%), nephrotoxicity (2%), and sensory neuropathy (4%). No grade >3 hemorrhagic events or hypertension cases were reported. OS was significantly higher in patients with the lowest TS mRNA levels [median, 29.6 months (95% CI, 26.2-32.9)] compared with those in patients with higher levels [median, 9.3 months (95% CI, 6.6-12.0); p = 0.0001]. TS expression (mRNA levels or protein expression) did not influence the treatment response.
Conclusion: Overall, PCB followed by maintenance pemetrexed and bevacizumab was effective and tolerable in Hispanic patients with non-squamous NSCLC. This regimen was associated with acceptable toxicity and prolonged OS, particularly in patients with low TS expression. We found a role for Ki67 and TS expression as prognostic factors.
Conflict of interest statement
Figures
Similar articles
-
Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer.J Clin Oncol. 2009 Jul 10;27(20):3284-9. doi: 10.1200/JCO.2008.20.8181. Epub 2009 May 11. J Clin Oncol. 2009. PMID: 19433684 Clinical Trial.
-
Maintenance therapy with pemetrexed and bevacizumab versus pemetrexed monotherapy after induction therapy with carboplatin, pemetrexed, and bevacizumab in patients with advanced non-squamous non small cell lung cancer.Eur J Cancer. 2016 May;58:30-7. doi: 10.1016/j.ejca.2016.01.013. Epub 2016 Feb 27. Eur J Cancer. 2016. PMID: 26922170 Clinical Trial.
-
A phase II study of carboplatin, pemetrexed, and bevacizumab followed by erlotinib and bevacizumab maintenance for non-squamous non-small cell lung cancer with wild-type EGFR (HOT1101).Int J Clin Oncol. 2018 Dec;23(6):1060-1069. doi: 10.1007/s10147-018-1318-z. Epub 2018 Jul 19. Int J Clin Oncol. 2018. PMID: 30027464 Clinical Trial.
-
The efficacy and toxicity of maintenance therapy with bevacizumab plus pemetrexed versus bevacizumab/pemetrexed alone for stage IIIB/IV nonsquamous non-small cell lung cancer: A meta-analysis of randomized controlled trials.J Clin Pharm Ther. 2022 Feb;47(2):157-167. doi: 10.1111/jcpt.13534. Epub 2021 Oct 6. J Clin Pharm Ther. 2022. PMID: 34617297 Review.
-
The Role of Combination Maintenance with Pemetrexed and Bevacizumab for Advanced Stage Nonsquamous Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis.Biomed Res Int. 2018 May 28;2018:5839081. doi: 10.1155/2018/5839081. eCollection 2018. Biomed Res Int. 2018. PMID: 29998136 Free PMC article.
Cited by
-
Immunotherapy at any line of treatment improves survival in patients with advanced metastatic non-small cell lung cancer (NSCLC) compared with chemotherapy (Quijote-CLICaP).Thorac Cancer. 2020 Feb;11(2):353-361. doi: 10.1111/1759-7714.13272. Epub 2019 Dec 12. Thorac Cancer. 2020. PMID: 31828967 Free PMC article.
-
Analysis of the relationship between Ki-67 expression and chemotherapy and prognosis in advanced non-small cell lung cancer.Transl Cancer Res. 2020 May;9(5):3491-3498. doi: 10.21037/tcr.2020.03.72. Transl Cancer Res. 2020. PMID: 35117714 Free PMC article.
-
Anlotinib as Maintenance Therapy After First-Line Chemotherapy Combined with Consolidation Radiation for Extensive-Stage Small Cell Lung Cancer.Technol Cancer Res Treat. 2025 Jan-Dec;24:15330338251317571. doi: 10.1177/15330338251317571. Technol Cancer Res Treat. 2025. PMID: 39887207 Free PMC article.
-
Augmented expression of Ki-67 is correlated with clinicopathological characteristics and prognosis for lung cancer patients: an up-dated systematic review and meta-analysis with 108 studies and 14,732 patients.Respir Res. 2018 Aug 13;19(1):150. doi: 10.1186/s12931-018-0843-7. Respir Res. 2018. PMID: 30103737 Free PMC article.
-
Current Smoking has a Detrimental Effect on Survival for Epidermal Growth Factor Receptor (EGFR) and Anaplastic Lymphoma Kinase (ALK) negative Advanced non-squamous Non-small Cell Lung Cancer (NSCLC) Patients Treated with Pemetrexed Continuation Maintenance.J Cancer. 2018 May 25;9(12):2140-2146. doi: 10.7150/jca.24872. eCollection 2018. J Cancer. 2018. PMID: 29937933 Free PMC article.
References
-
- Cancer IARC. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. In, 2012.
-
- Scrump DC DK CR, Marks LB Giaccone G. Non-Small Cell Lung Cancer In: DeVita VT L T, Rosenberg SA editor Cancer: Principles and Practice of Oncology. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins, 2011.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

