PEGylation of Truncated Streptokinase Leads to Formulation of a Useful Drug with Ameliorated Attributes
- PMID: 27192220
- PMCID: PMC4871584
- DOI: 10.1371/journal.pone.0155831
PEGylation of Truncated Streptokinase Leads to Formulation of a Useful Drug with Ameliorated Attributes
Abstract
Streptokinase (SK) remains a favored thrombolytic agent in the developing world as compared to the nearly 10-fold more expensive human tissue-plasminogen activator (tPA) for the dissolution of pathological fibrin clots in myocardial infarction. However, unlike the latter, SK induces systemic activation of plasmin which results in a greater risk of hemorrhage. Being of bacterial origin, it elicits generation of unwanted antibody and has a relatively short half-life in vivo that needs to be addressed to make it more efficacious clinically. In order to address these lacunae, in the present study we have incorporated cysteine residues specifically at the N- and C-termini of partially truncated SK and these were then PEGylated successfully. Some of the obtained derivatives displayed enhanced plasmin resistance, longer half-life (upto several hours), improved fibrin clot-specificity and reduced immune-reactivity as compared to the native SK (nSK). This paves the way for devising next-generation SK-based thrombolytic agent/s that besides being fibrin clot-specific are endowed with an improved efficacy by virtue of an extended in vivo half-life.
Conflict of interest statement
Figures
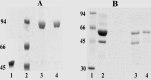

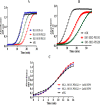
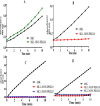




References
-
- Collen D. Coronary thrombolysis: streptokinase or recombinant tissue-type plasminogen activator? Annals of internal medicine. 1990;112(7):529–38. - PubMed
-
- Reddy KNN, Markus G. Mechanism of activation of human plasminogen by streptokinase Presence of active center in streptokinase-plasminogen complex. Journal of Biological Chemistry. 1972;247(6):1683–91. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

