The Chlamydia muridarum Organisms Fail to Auto-Inoculate the Mouse Genital Tract after Colonization in the Gastrointestinal Tract for 70 days
- PMID: 27192556
- PMCID: PMC4871562
- DOI: 10.1371/journal.pone.0155880
The Chlamydia muridarum Organisms Fail to Auto-Inoculate the Mouse Genital Tract after Colonization in the Gastrointestinal Tract for 70 days
Abstract
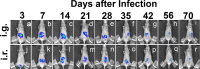
Chlamydia muridarum is known to colonize in the gastrointestinal tract for long periods of time, which has been hypothesized to serve as a reservoir for spreading to the genital tract. To test this hypothesis, a luciferase-expressing C. muridarum was used to establish a long-lasting infection in the mouse gastrointestinal tract following either intragastric or intrarectal inoculations. In vivo imaging revealed significant bioluminescent signals mainly in the mouse abdominal area throughout the experiments. Ex vivo imaging localized the signals to the mouse gastrointestinal tract, which was confirmed by monitoring the C. muridarum organisms in the mouse organs/tissues. Despite the long-lasting colonization in the gastrointestinal tract and active shedding of infectious organisms in the rectal swabs, the organisms did not cause any significant infection or pathology in the genital tract throughout the experiments, which was reproduced in multiple strains of mice and with an increased inoculation dose to the gastrointestinal tract. The above observations have demonstrated that the long-lasting C. muridarum organisms from the gastrointestinal tract are inefficient in auto-inoculating the genital tract, suggesting that the gastrointestinal tract Chlamydia may utilize an indirect mechanism to affect its pathogenicity in the genital tract.
Conflict of interest statement
Figures






References
-
- Campos-Hernandez E, Vazquez-Chagoyan JC, Salem AZ, Saltijeral-Oaxaca JA, Escalante-Ochoa C, Lopez-Heydeck SM, et al. Prevalence and molecular identification of Chlamydia abortus in commercial dairy goat farms in a hot region in Mexico. Tropical animal health and production. 2014;46(6):919–24. 10.1007/s11250-014-0585-6 . - DOI - PubMed
-
- Gratrix J, Singh AE, Bergman J, Egan C, McGinnis J, Drews SJ, et al. Prevalence and characteristics of rectal chlamydia and gonorrhea cases among men who have sex with men after the introduction of nucleic acid amplification test screening at 2 Canadian sexually transmitted infection clinics. Sex Transm Dis. 2014;41(10):589–91. 10.1097/OLQ.0000000000000176 . - DOI - PubMed
-
- Gratrix J, Singh AE, Bergman J, Egan C, Plitt SS, McGinnis J, et al. Evidence for increased Chlamydia case finding after the introduction of rectal screening among women attending 2 Canadian sexually transmitted infection clinics. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015;60(3):398–404. 10.1093/cid/ciu831 . - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

