Ethylene is involved in root phosphorus remobilization in rice (Oryza sativa) by regulating cell-wall pectin and enhancing phosphate translocation to shoots
- PMID: 27192711
- PMCID: PMC5055617
- DOI: 10.1093/aob/mcw044
Ethylene is involved in root phosphorus remobilization in rice (Oryza sativa) by regulating cell-wall pectin and enhancing phosphate translocation to shoots
Abstract
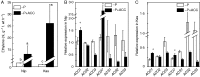
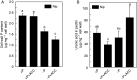
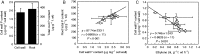
Background and aims Plants are able to grow under phosphorus (P)-deficient conditions by coordinating Pi acquisition, translocation from roots to shoots and remobilization within the plant. Previous reports have demonstrated that cell-wall pectin contributes greatly to rice cell-wall Pi re-utilization under P-deficient conditions, but whether other factors such as ethylene also affect the pectin-remobilizing capacity remains unclear. Methods Two rice cultivars, 'Nipponbare' (Nip) and 'Kasalath' (Kas) were cultured in the +P (complete nutrient solution), -P (withdrawing P from the complete nutrient solution), +P+ACC (1-amino-cyclopropane-1-carboxylic acid, an ethylene precursor, adding 1 μm ACC to the complete nutrient solution) and -P+ACC (adding 1 μm ACC to -P nutrient solution) nutrient solutions for 7 d. Key Results After 7 d -P treatment, there was clearly more soluble P in Nip root and shoot, accompanied by additional production of ethylene in Nip root compared with Kas. Under P-deficient conditions, addition of ACC significantly increased the cell-wall pectin content and decreased cell-wall retained P, and thus more soluble P was released to the root and translocated to the shoot, which was mediated by the expression of the P deficiency-responsive gene OsPT2, which also strongly induced by ACC treatment under both P-sufficient and P-deficient conditions. Conclusions Ethylene positively regulates pectin content and expression of OsPT2, which ultimately makes more P available by facilitating the solubilization of P fixed in the cell wall and its translocation to the shoot.
Keywords: Rice; cell-wall polysaccharides; ethylene; gene expression; pectin; phosphorus; remobilization; transport.
© The Author 2016. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures





Similar articles
-
Nitric oxide acts upstream of ethylene in cell wall phosphorus reutilization in phosphorus-deficient rice.J Exp Bot. 2017 Jan 1;68(3):753-760. doi: 10.1093/jxb/erw480. J Exp Bot. 2017. PMID: 28064177 Free PMC article.
-
Pectin enhances rice (Oryza sativa) root phosphorus remobilization.J Exp Bot. 2015 Feb;66(3):1017-24. doi: 10.1093/jxb/eru461. Epub 2014 Dec 20. J Exp Bot. 2015. PMID: 25528599
-
Nitrate inhibits the remobilization of cell wall phosphorus under phosphorus-starvation conditions in rice (Oryza sativa).Planta. 2018 Jul;248(1):185-196. doi: 10.1007/s00425-018-2892-z. Epub 2018 Apr 16. Planta. 2018. PMID: 29663070
-
Enhancing phosphorus and zinc acquisition efficiency in rice: a critical review of root traits and their potential utility in rice breeding.Ann Bot. 2013 Jul;112(2):331-45. doi: 10.1093/aob/mcs217. Epub 2012 Oct 15. Ann Bot. 2013. PMID: 23071218 Free PMC article. Review.
-
Adaptive strategies of plants to conserve internal phosphorus under P deficient condition to improve P utilization efficiency.Physiol Mol Biol Plants. 2022 Dec;28(11-12):1981-1993. doi: 10.1007/s12298-022-01255-8. Epub 2022 Dec 5. Physiol Mol Biol Plants. 2022. PMID: 36573147 Free PMC article. Review.
Cited by
-
Editing of the OsACS locus alters phosphate deficiency-induced adaptive responses in rice seedlings.J Exp Bot. 2019 Mar 27;70(6):1927-1940. doi: 10.1093/jxb/erz074. J Exp Bot. 2019. PMID: 30810167 Free PMC article.
-
Multiple analysis of root exudates and microbiome in rice (Oryza sativa) under low P conditions.Arch Microbiol. 2021 Nov;203(9):5599-5611. doi: 10.1007/s00203-021-02539-5. Epub 2021 Aug 28. Arch Microbiol. 2021. PMID: 34455446
-
Nitric oxide acts upstream of ethylene in cell wall phosphorus reutilization in phosphorus-deficient rice.J Exp Bot. 2017 Jan 1;68(3):753-760. doi: 10.1093/jxb/erw480. J Exp Bot. 2017. PMID: 28064177 Free PMC article.
-
Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of apple dwarfing rootstock root morphogenesis under nitrogen and/or phosphorus deficient conditions.Front Plant Sci. 2023 Jun 19;14:1120777. doi: 10.3389/fpls.2023.1120777. eCollection 2023. Front Plant Sci. 2023. PMID: 37404544 Free PMC article.
-
Exogenously applied spermidine alleviates hypoxia stress in Phyllostachys praecox seedlings via changes in endogenous hormones and gene expression.BMC Plant Biol. 2022 Apr 19;22(1):200. doi: 10.1186/s12870-022-03568-y. BMC Plant Biol. 2022. PMID: 35439921 Free PMC article.
References
-
- Ae N, Arihara J, Okada K, Yoshihara T, Johansen C. 1990. Phosphorus uptake by pigeonpea and its role in cropping systems of Indian subcontinent. Science 248: 477–480. - PubMed
-
- Ae N, Otani T, Makino T, Tazawa J. 1996. Role of cell wall of groundnut roots in solubilizing sparingly soluble phosphorus in soil. Plant and Soil 186: 197–204.
-
- Ai PH, Sun SB, Zhao JN, et al. 2009. Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. The Plant Journal 57: 798–809. - PubMed
-
- Blamey FPC, Edmeades DC, Wheeler DM. 1990. Role of root cation-exchange capacity in differential aluminum tolerance of Lotus species. Journal of Plant Nutrition 13: 729–744.
-
- Blumenkrantz N, Asboe-Hansen G. 1973. New method for quantitative determination of uronic acids. Analytical Biochemistry 54: 484–489. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

