PPAR-α, a lipid-sensing transcription factor, regulates blood-brain barrier efflux transporter expression
- PMID: 27193034
- PMCID: PMC5453444
- DOI: 10.1177/0271678X16650216
PPAR-α, a lipid-sensing transcription factor, regulates blood-brain barrier efflux transporter expression
Abstract
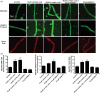
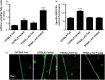
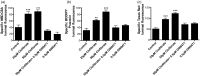
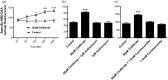
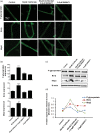
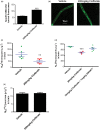
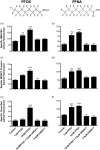
Lipid sensor peroxisome proliferator-activated receptor alpha (PPAR- α) is the master regulator of lipid metabolism. Dietary release of endogenous free fatty acids, fibrates, and certain persistent environmental pollutants, e.g. perfluoroalkyl fire-fighting foam components, are peroxisome proliferator-activated receptor alpha ligands. Here, we define a role for peroxisome proliferator-activated receptor alpha in regulating the expression of three ATP-driven drug efflux transporters at the rat and mouse blood-brain barriers: P-glycoprotein (Abcb1), breast cancer resistance protein (Bcrp/Abcg2), and multidrug resistance-associated protein 2 (Mrp2/Abcc2). Exposing isolated rat brain capillaries to linoleic acid, clofibrate, or PKAs increased the transport activity and protein expression of the three ABC transporters. These effects were blocked by the PPAR- α antagonist, GW6471. Dosing rats with 20 mg/kg or 200 mg/kg of clofibrate decreased the brain accumulation of the P-glycoprotein substrate, verapamil, by 50% (in situ brain perfusion; effects blocked by GW6471) and increased P-glycoprotein expression and activity in capillaries ex vivo. Fasting C57Bl/6 wild-type mice for 24 h increased both serum lipids and brain capillary P-glycoprotein transport activity. Fasting did not alter P-glycoprotein activity in PPAR- α knockout mice. These results indicate that hyperlipidemia, lipid-lowering fibrates and exposure to certain fire-fighting foam components activate blood-brain barrier peroxisome proliferator-activated receptor alpha, increase drug efflux transporter expression and reduce drug delivery to the brain.
Keywords: Blood–brain barrier; P-glycoprotein; PPAR; fasting; fibrate.
Figures
Similar articles
-
In vivo and ex vivo regulation of breast cancer resistant protein (Bcrp) by peroxisome proliferator-activated receptor alpha (Pparα) at the blood-brain barrier.J Neurochem. 2015 Dec;135(6):1113-22. doi: 10.1111/jnc.13389. Epub 2015 Nov 13. J Neurochem. 2015. PMID: 26465636 Free PMC article.
-
Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood-brain and blood-spinal cord barriers.J Neurosci. 2014 Jun 18;34(25):8585-93. doi: 10.1523/JNEUROSCI.2935-13.2014. J Neurosci. 2014. PMID: 24948812 Free PMC article.
-
Constitutive androstane receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier.Mol Pharmacol. 2010 Sep;78(3):376-83. doi: 10.1124/mol.110.063685. Epub 2010 Jun 14. Mol Pharmacol. 2010. PMID: 20547735 Free PMC article.
-
Regulation of ABC transporters blood-brain barrier: the good, the bad, and the ugly.Adv Cancer Res. 2015;125:43-70. doi: 10.1016/bs.acr.2014.10.002. Epub 2015 Jan 8. Adv Cancer Res. 2015. PMID: 25640266 Review.
-
Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense.Toxicol Appl Pharmacol. 2005 May 1;204(3):216-37. doi: 10.1016/j.taap.2004.10.012. Toxicol Appl Pharmacol. 2005. PMID: 15845415 Review.
Cited by
-
Current advancements and future perspectives of long noncoding RNAs in lipid metabolism and signaling.J Adv Res. 2023 Jun;48:105-123. doi: 10.1016/j.jare.2022.08.007. Epub 2022 Aug 13. J Adv Res. 2023. PMID: 35973552 Free PMC article. Review.
-
Rosiglitazone ameliorates tissue plasminogen activator-induced brain hemorrhage after stroke.CNS Neurosci Ther. 2019 Dec;25(12):1343-1352. doi: 10.1111/cns.13260. Epub 2019 Nov 22. CNS Neurosci Ther. 2019. PMID: 31756041 Free PMC article.
-
Dual PPAR delta/gamma agonists offer therapeutic potential for Alzheimer's disease.Neural Regen Res. 2024 Jun 1;19(6):1175-1176. doi: 10.4103/1673-5374.386410. Epub 2023 Oct 2. Neural Regen Res. 2024. PMID: 37905851 Free PMC article. No abstract available.
-
MG53 Protects against Sepsis-Induced Myocardial Dysfunction by Upregulating Peroxisome Proliferator-Activated Receptor-α.Oxid Med Cell Longev. 2020 Aug 27;2020:7413693. doi: 10.1155/2020/7413693. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32908637 Free PMC article.
-
Organic Cation Transporter (OCT/OCTN) Expression at Brain Barrier Sites: Focus on CNS Drug Delivery.Handb Exp Pharmacol. 2021;266:301-328. doi: 10.1007/164_2021_448. Handb Exp Pharmacol. 2021. PMID: 33674914 Free PMC article.
References
-
- Miller DS, Nobmann SN, Gutmann H, et al. Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol 2000; 58: 1357–1367. - PubMed
-
- Hartz AM, Bauer B, Fricker G, Miller DS. Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol Pharmacol 2004; 66: 387–394. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

