Mobile Technology for Vegetable Consumption: A Randomized Controlled Pilot Study in Overweight Adults
- PMID: 27193036
- PMCID: PMC4889871
- DOI: 10.2196/mhealth.5146
Mobile Technology for Vegetable Consumption: A Randomized Controlled Pilot Study in Overweight Adults
Abstract
Background: Mobile apps present a potentially cost-effective tool for delivering behavior change interventions at scale, but no known studies have tested the efficacy of apps as a tool to specifically increase vegetable consumption among overweight adults.
Objective: The purpose of this pilot study was to assess the initial efficacy and user acceptability of a theory-driven mobile app to increase vegetable consumption.
Methods: A total of 17 overweight adults aged 42.0 (SD 7.3) years with a body mass index (BMI) of 32.0 (SD 3.5) kg/m(2) were randomized to the use of Vegethon (a fully automated theory-driven mobile app enabling self-monitoring of vegetable consumption, goal setting, feedback, and social comparison) or a wait-listed control condition. All participants were recruited from an ongoing 12-month weight loss trial (parent trial). Researchers who performed data analysis were blinded to condition assignment. The primary outcome measure was daily vegetable consumption, assessed using an adapted version of the validated Harvard Food Frequency Questionnaire administered at baseline and 12 weeks after randomization. An analysis of covariance was used to assess differences in 12-week vegetable consumption between intervention and control conditions, controlling for baseline. App usability and satisfaction were measured via a 21-item post-intervention questionnaire.
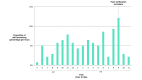
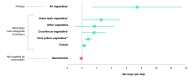
Results: Using intention-to-treat analyses, all enrolled participants (intervention: 8; control: 9) were analyzed. Of the 8 participants randomized to the intervention, 5 downloaded the app and logged their vegetable consumption a mean of 0.7 (SD 0.9) times per day, 2 downloaded the app but did not use it, and 1 never downloaded it. Consumption of vegetables was significantly greater among the intervention versus control condition at the end of the 12-week pilot study (adjusted mean difference: 7.4 servings; 95% CI 1.4-13.5; P=.02). Among secondary outcomes defined a priori, there was significantly greater consumption of green leafy vegetables, cruciferous vegetables, and dark yellow vegetables (adjusted mean difference: 2.6, 1.6, and 0.8 servings; 95% CI 0.1-5.0, 0.1-3.2, and 0.3-1.4; P=.04, P=.04, and P=.004, respectively). Participants reported positive experiences with the app, including strong agreement with the statements "I have found Vegethon easy to use" and "I would recommend Vegethon to a friend" (mean 4.6 (SD 0.6) and 4.2 (SD 0.8), respectively, (on a 5-point scale).
Conclusions: Vegethon demonstrated initial efficacy and user acceptability. A mobile app intervention may be useful for increasing vegetable consumption among overweight adults. The small sample size prevented precise estimates of effect sizes. Given the improved health outcomes associated with increases in vegetable consumption, these findings indicate the need for larger, longer-term evaluations of Vegethon and similar technologies among overweight adults and other suitable target groups.
Trial registration: ClinicalTrials.gov NCT01826591; https://clinicaltrials.gov/ct2/show/NCT01826591 (Archived by WebCite at http://www.webcitation.org/6hYDw2AOB).
Keywords: cell phones; diet; eating; health behavior; pilot projects; randomized controlled trial; telemedicine; vegetables.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures




References
-
- Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J Nutr. 2006 Oct;136(10):2588–93.136/10/2588 - PubMed
-
- Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Colditz G, Ascherio A, Rosner B, Spiegelman D, Willett WC. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001 Jun 19;134(12):1106–14.200106190-00010 - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

