Virtual-tissue computer simulations define the roles of cell adhesion and proliferation in the onset of kidney cystic disease
- PMID: 27193300
- PMCID: PMC5221598
- DOI: 10.1091/mbc.E16-01-0059
Virtual-tissue computer simulations define the roles of cell adhesion and proliferation in the onset of kidney cystic disease
Abstract
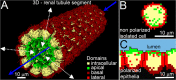
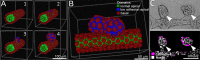
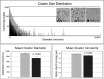
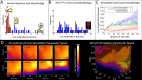
In autosomal dominant polycystic kidney disease (ADPKD), cysts accumulate and progressively impair renal function. Mutations in PKD1 and PKD2 genes are causally linked to ADPKD, but how these mutations drive cell behaviors that underlie ADPKD pathogenesis is unknown. Human ADPKD cysts frequently express cadherin-8 (cad8), and expression of cad8 ectopically in vitro suffices to initiate cystogenesis. To explore cell behavioral mechanisms of cad8-driven cyst initiation, we developed a virtual-tissue computer model. Our simulations predicted that either reduced cell-cell adhesion or reduced contact inhibition of proliferation triggers cyst induction. To reproduce the full range of cyst morphologies observed in vivo, changes in both cell adhesion and proliferation are required. However, only loss-of-adhesion simulations produced morphologies matching in vitro cad8-induced cysts. Conversely, the saccular cysts described by others arise predominantly by decreased contact inhibition, that is, increased proliferation. In vitro experiments confirmed that cell-cell adhesion was reduced and proliferation was increased by ectopic cad8 expression. We conclude that adhesion loss due to cadherin type switching in ADPKD suffices to drive cystogenesis. Thus, control of cadherin type switching provides a new target for therapeutic intervention.
© 2016 Belmonte, Clendenon, Oliveira, et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures


References
-
- Aguiari G, Campanella M, Manzati E, Pinton P, Banzi M, Moretti S, Piva R, Rizzuto R, del Senno L. Expression of polycystin-1 C-terminal fragment enhances the ATP-induced Ca2+ release in human kidney cells. Biochem Biophys Res Commun. 2003;301:657–664. - PubMed
-
- Bacallao RL, McNeill H. Cystic kidney diseases and planar cell polarity signaling. Clin Genet. 2009;75:107–117. - PubMed
-
- Baert L. Hereditary polycystic kidney disease (adult form): a microdissection study of two cases at an early stage of the disease. Kidney Int. 1978;13:519–525. - PubMed
-
- Baert L, Steg A. On the pathogenesis of simple renal cysts in the adult. A microdissection study. Urol Res. 1977;5:103–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

