An obligatory role for neurotensin in high-fat-diet-induced obesity
- PMID: 27193687
- PMCID: PMC5484414
- DOI: 10.1038/nature17662
An obligatory role for neurotensin in high-fat-diet-induced obesity
Abstract
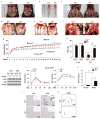
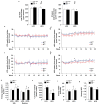
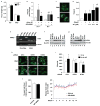
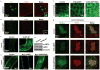
Obesity and its associated comorbidities (for example, diabetes mellitus and hepatic steatosis) contribute to approximately 2.5 million deaths annually and are among the most prevalent and challenging conditions confronting the medical profession. Neurotensin (NT; also known as NTS), a 13-amino-acid peptide predominantly localized in specialized enteroendocrine cells of the small intestine and released by fat ingestion, facilitates fatty acid translocation in rat intestine, and stimulates the growth of various cancers. The effects of NT are mediated through three known NT receptors (NTR1, 2 and 3; also known as NTSR1, 2, and NTSR3, respectively). Increased fasting plasma levels of pro-NT (a stable NT precursor fragment produced in equimolar amounts relative to NT) are associated with increased risk of diabetes, cardiovascular disease and mortality; however, a role for NT as a causative factor in these diseases is unknown. Here we show that NT-deficient mice demonstrate significantly reduced intestinal fat absorption and are protected from obesity, hepatic steatosis and insulin resistance associated with high fat consumption. We further demonstrate that NT attenuates the activation of AMP-activated protein kinase (AMPK) and stimulates fatty acid absorption in mice and in cultured intestinal cells, and that this occurs through a mechanism involving NTR1 and NTR3 (also known as sortilin). Consistent with the findings in mice, expression of NT in Drosophila midgut enteroendocrine cells results in increased lipid accumulation in the midgut, fat body, and oenocytes (specialized hepatocyte-like cells) and decreased AMPK activation. Remarkably, in humans, we show that both obese and insulin-resistant subjects have elevated plasma concentrations of pro-NT, and in longitudinal studies among non-obese subjects, high levels of pro-NT denote a doubling of the risk of developing obesity later in life. Our findings directly link NT with increased fat absorption and obesity and suggest that NT may provide a prognostic marker of future obesity and a potential target for prevention and treatment.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


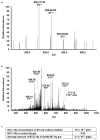
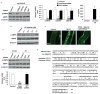
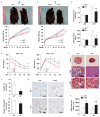

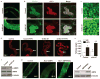
Comment in
-
Obesity: Could 13 amino acids combat obesity?Nat Rev Endocrinol. 2016 Jul;12(7):371. doi: 10.1038/nrendo.2016.83. Epub 2016 May 27. Nat Rev Endocrinol. 2016. PMID: 27230952 No abstract available.
References
-
- Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. - PubMed
-
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. - PubMed
-
- Polak JM, et al. Specific localisation of neurotensin to the N cell in human intestine by radioimmunoassay and immunocytochemistry. Nature. 1977;270:183–184. - PubMed
-
- Ferris CF, Hammer RA, Leeman SE. Elevation of plasma neurotensin during lipid perfusion of rat small intestine. Peptides. 1981;2(Suppl 2):263–266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AG010885/AG/NIA NIH HHS/United States
- R01 GM079684/GM/NIGMS NIH HHS/United States
- R01 ES022191/ES/NIEHS NIH HHS/United States
- T32 CA165990/CA/NCI NIH HHS/United States
- T32 CA160003/CA/NCI NIH HHS/United States
- P01 CA163223/CA/NCI NIH HHS/United States
- R01 HL120507/HL/NHLBI NIH HHS/United States
- R37 AG10885/AG/NIA NIH HHS/United States
- 282255/ERC_/European Research Council/International
- R01 HL12050/HL/NHLBI NIH HHS/United States
- P20 GM103527/GM/NIGMS NIH HHS/United States
- R01 NS077284/NS/NINDS NIH HHS/United States
- R01 HL073085/HL/NHLBI NIH HHS/United States
- S10 RR029127/RR/NCRR NIH HHS/United States
- P30 CA177558/CA/NCI NIH HHS/United States
- U24 DK097215/DK/NIDDK NIH HHS/United States
- R01 DK048498/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

