Benefit-risk Evaluation for Diagnostics: A Framework (BED-FRAME)
- PMID: 27193750
- PMCID: PMC4996133
- DOI: 10.1093/cid/ciw329
Benefit-risk Evaluation for Diagnostics: A Framework (BED-FRAME)
Abstract
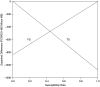
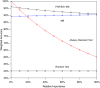
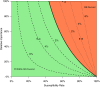
The medical community needs systematic and pragmatic approaches for evaluating the benefit-risk trade-offs of diagnostics that assist in medical decision making. Benefit-Risk Evaluation of Diagnostics: A Framework (BED-FRAME) is a strategy for pragmatic evaluation of diagnostics designed to supplement traditional approaches. BED-FRAME evaluates diagnostic yield and addresses 2 key issues: (1) that diagnostic yield depends on prevalence, and (2) that different diagnostic errors carry different clinical consequences. As such, evaluating and comparing diagnostics depends on prevalence and the relative importance of potential errors. BED-FRAME provides a tool for communicating the expected clinical impact of diagnostic application and the expected trade-offs of diagnostic alternatives. BED-FRAME is a useful fundamental supplement to the standard analysis of diagnostic studies that will aid in clinical decision making.
Keywords: benefit-risk; diagnostic yield; diagnostics; pragmatism.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures

References
-
- Gelber RD, Gelman RS, Goldhirsch A. A quality-of-life oriented endpoint for comparing treatments. Biometrics 1989; 45:781–95. - PubMed
-
- Chuang-Stein C, Mohberg NR, Sinkula MS. Three measures for simultaneously evaluating benefits and risks using categorical data from clinical trials. Stat Med 1991; 10:1349–59. - PubMed
-
- Follmann D. Regression analysis based on pairwise ordering of patients’ clinical histories. Stat Med 2002; 21:3353–67. - PubMed
-
- Pocock SJ, Ariti CA, Collier TJ, Wang D. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur Heart J 2012; 33:176–82. - PubMed
-
- Egan JP. Signal detection theory and ROC analysis. New York: Academic Press, 1975.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

