Development and evaluation of two subunit vaccine candidates containing antigens of hepatitis E virus, rotavirus, and astrovirus
- PMID: 27194006
- PMCID: PMC4872161
- DOI: 10.1038/srep25735
Development and evaluation of two subunit vaccine candidates containing antigens of hepatitis E virus, rotavirus, and astrovirus
Abstract
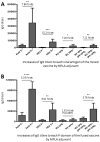
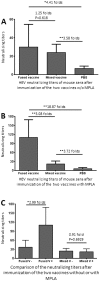
Hepatitis E virus (HEV), rotavirus (RV), and astrovirus (AstV) are important pathogens that transmit through a common fecal-oral route, causing hepatitis (HEV) and gastroenteritis (RV and AstV) respectively in humans. In this study, we developed and evaluated two subunit vaccine candidates that consisted of the same protruding or spike protein antigens of the three viruses in two formats, a fusion of the three antigens into one molecule (fused vaccine) vs. a mixture of the three free antigens together (mixed vaccine). Both vaccines were easily made via E. coli expression system. Mouse immunization experiments showed that the fused vaccine elicited significantly higher antibody responses against the three viral antigens than those induced by the mixed vaccine. In addition, the mouse post-immune antisera of the fused vaccine revealed significantly higher neutralizing titers against HEV infection in cell culture, as well as significantly higher 50% blocking titers (BT50) against RV VP8-HBGA receptor interactions than those of the post-immune antisera after immunization of the mixed vaccine. Thus, the fused vaccine is a promising trivalent vaccine candidate against HEV, RV, and AstV, which is worth for further development.
Figures




Similar articles
-
A trivalent vaccine candidate against hepatitis E virus, norovirus, and astrovirus.Vaccine. 2016 Feb 10;34(7):905-13. doi: 10.1016/j.vaccine.2015.12.068. Epub 2016 Jan 9. Vaccine. 2016. PMID: 26778421 Free PMC article.
-
A dual vaccine candidate against norovirus and hepatitis E virus.Vaccine. 2014 Jan 16;32(4):445-52. doi: 10.1016/j.vaccine.2013.11.064. Epub 2013 Nov 27. Vaccine. 2014. PMID: 24291540 Free PMC article.
-
Rectal immunization with rotavirus virus-like particles induces systemic and mucosal humoral immune responses and protects mice against rotavirus infection.J Virol. 2006 Feb;80(4):1752-61. doi: 10.1128/JVI.80.4.1752-1761.2006. J Virol. 2006. PMID: 16439532 Free PMC article.
-
Vaccines for viral and bacterial pathogens causing acute gastroenteritis: Part I: Overview, vaccines for enteric viruses and Vibrio cholerae.Hum Vaccin Immunother. 2015;11(3):584-600. doi: 10.1080/21645515.2015.1011019. Hum Vaccin Immunother. 2015. PMID: 25715048 Free PMC article. Review.
-
Rotavirus VP6 preparations as a non-replicating vaccine candidates.Vaccine. 2015 Jun 26;33(29):3281-7. doi: 10.1016/j.vaccine.2015.05.026. Epub 2015 May 26. Vaccine. 2015. PMID: 26021725 Review.
Cited by
-
Astrovirus Infection and Diarrhea in 8 Countries.Pediatrics. 2018 Jan;141(1):e20171326. doi: 10.1542/peds.2017-1326. Epub 2017 Dec 19. Pediatrics. 2018. PMID: 29259078 Free PMC article.
-
Immune response and protective efficacy of the S particle presented rotavirus VP8* vaccine in mice.Vaccine. 2019 Jul 9;37(30):4103-4110. doi: 10.1016/j.vaccine.2019.05.075. Epub 2019 Jun 11. Vaccine. 2019. PMID: 31201052 Free PMC article.
-
Epidemiology of Classic and Novel Human Astrovirus: Gastroenteritis and Beyond.Viruses. 2017 Feb 18;9(2):33. doi: 10.3390/v9020033. Viruses. 2017. PMID: 28218712 Free PMC article. Review.
-
Pseudovirus Nanoparticles Displaying Plasmodium Circumsporozoite Proteins Elicited High Titers of Sporozoite-Binding Antibody.Vaccines (Basel). 2023 Oct 27;11(11):1650. doi: 10.3390/vaccines11111650. Vaccines (Basel). 2023. PMID: 38005982 Free PMC article.
-
Histo-blood group antigens as receptors for rotavirus, new understanding on rotavirus epidemiology and vaccine strategy.Emerg Microbes Infect. 2017 Apr 12;6(4):e22. doi: 10.1038/emi.2017.30. Emerg Microbes Infect. 2017. PMID: 28400594 Free PMC article. Review.
References
-
- Panda S. K., Thakral D. & Rehman S. Hepatitis E virus. Reviews in medical virology 17, 151–180 (2007). - PubMed
-
- Kumar S., Subhadra S., Singh B. & Panda B. K. Hepatitis E virus: the current scenario. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases 17, e228–233 (2013). - PubMed
-
- Glass R. I. et al. The changing epidemiology of astrovirus-associated gastroenteritis: a review. Archives of virology. Supplementum 12, 287–300 (1996). - PubMed
-
- Tate J. E. et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 12, 136–141 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

