The Oxygen Dilemma: A Severe Challenge for the Application of Monooxygenases?
- PMID: 27194219
- PMCID: PMC5096067
- DOI: 10.1002/cbic.201600176
The Oxygen Dilemma: A Severe Challenge for the Application of Monooxygenases?
Abstract
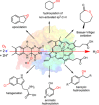
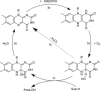
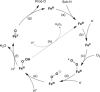
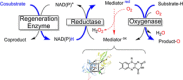
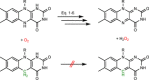
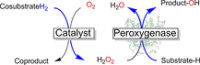
Monooxygenases are promising catalysts because they in principle enable the organic chemist to perform highly selective oxyfunctionalisation reactions that are otherwise difficult to achieve. For this, monooxygenases require reducing equivalents, to allow reductive activation of molecular oxygen at the enzymes' active sites. However, these reducing equivalents are often delivered to O2 either directly or via a reduced intermediate (uncoupling), yielding hazardous reactive oxygen species and wasting valuable reducing equivalents. The oxygen dilemma arises from monooxygenases' dependency on O2 and the undesired uncoupling reaction. With this contribution we hope to generate a general awareness of the oxygen dilemma and to discuss its nature and some promising solutions.
Keywords: biocatalysis; monooxygenases; oxidoreductases; oxyfunctionalization; uncoupling.
© 2016 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures

Similar articles
-
Developing mononuclear copper-active-oxygen complexes relevant to reactive intermediates of biological oxidation reactions.Acc Chem Res. 2015 Jul 21;48(7):2066-74. doi: 10.1021/acs.accounts.5b00140. Epub 2015 Jun 18. Acc Chem Res. 2015. PMID: 26086527
-
Coupling Oxygen Consumption with Hydrocarbon Oxidation in Bacterial Multicomponent Monooxygenases.Acc Chem Res. 2015 Sep 15;48(9):2632-9. doi: 10.1021/acs.accounts.5b00312. Epub 2015 Aug 21. Acc Chem Res. 2015. PMID: 26293615 Free PMC article.
-
Flavoprotein monooxygenases: Versatile biocatalysts.Biotechnol Adv. 2021 Nov 1;51:107712. doi: 10.1016/j.biotechadv.2021.107712. Epub 2021 Feb 13. Biotechnol Adv. 2021. PMID: 33588053 Review.
-
Flavin-N5OOH Functions as both a Powerful Nucleophile and a Base in the Superfamily of Flavoenzymes.Angew Chem Int Ed Engl. 2024 Apr 2;63(14):e202318629. doi: 10.1002/anie.202318629. Epub 2024 Feb 19. Angew Chem Int Ed Engl. 2024. PMID: 38299700
-
Control of catalysis in flavin-dependent monooxygenases.Arch Biochem Biophys. 2010 Jan 1;493(1):26-36. doi: 10.1016/j.abb.2009.11.028. Epub 2009 Nov 26. Arch Biochem Biophys. 2010. PMID: 19944667 Review.
Cited by
-
Towards electroenzymatic processes involving old yellow enzymes and mediated cofactor regeneration.Eng Life Sci. 2016 Oct 12;17(1):71-76. doi: 10.1002/elsc.201600158. eCollection 2017 Jan. Eng Life Sci. 2016. PMID: 32624730 Free PMC article.
-
Living with Oxygen.Acc Chem Res. 2018 Aug 21;51(8):1850-1857. doi: 10.1021/acs.accounts.8b00245. Epub 2018 Jul 17. Acc Chem Res. 2018. PMID: 30016077 Free PMC article.
-
Catalytic Oxygenation of Hydrocarbons by Mono-μ-oxo Dicopper(II) Species Resulting from O-O Cleavage of Tetranuclear CuI /CuII Peroxo Complexes.Angew Chem Int Ed Engl. 2021 Jun 14;60(25):14154-14162. doi: 10.1002/anie.202101035. Epub 2021 May 7. Angew Chem Int Ed Engl. 2021. PMID: 33856088 Free PMC article.
-
Hydroxyl Radical-Coupled Electron-Transfer Mechanism of Flavin-Dependent Hydroxylases.J Phys Chem B. 2019 Sep 26;123(38):8065-8073. doi: 10.1021/acs.jpcb.9b08178. Epub 2019 Sep 18. J Phys Chem B. 2019. PMID: 31532200 Free PMC article.
-
Morpholine-based buffers activate aerobic photobiocatalysis via spin correlated ion pair formation.Catal Sci Technol. 2019 Mar 21;9(6):1365-1371. doi: 10.1039/c8cy02524j. Epub 2019 Feb 11. Catal Sci Technol. 2019. PMID: 31131076 Free PMC article.
References
-
- None
-
- Bornscheuer U. T., Huisman G. W., Kazlauskas R. J., Lutz S., Moore J. C., Robins K., Nature 2012, 485, 185–194; - PubMed
-
- Hollmann F., Arends I. W. C. E., Holtmann D., Green Chem. 2011, 13, 2285–2313;
-
- Hollmann F., Arends I. W. C. E., Buehler K., Schallmey A., Buhler B., Green Chem. 2011, 13, 226–265.
-
- None
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

