Multimodal Feature Integration in the Angular Gyrus during Episodic and Semantic Retrieval
- PMID: 27194327
- PMCID: PMC4871983
- DOI: 10.1523/JNEUROSCI.4310-15.2016
Multimodal Feature Integration in the Angular Gyrus during Episodic and Semantic Retrieval
Abstract
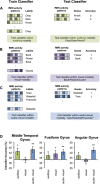
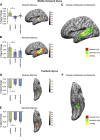
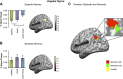
Much evidence from distinct lines of investigation indicates the involvement of angular gyrus (AnG) in the retrieval of both episodic and semantic information, but the region's precise function and whether that function differs across episodic and semantic retrieval have yet to be determined. We used univariate and multivariate fMRI analysis methods to examine the role of AnG in multimodal feature integration during episodic and semantic retrieval. Human participants completed episodic and semantic memory tasks involving unimodal (auditory or visual) and multimodal (audio-visual) stimuli. Univariate analyses revealed the recruitment of functionally distinct AnG subregions during the retrieval of episodic and semantic information. Consistent with a role in multimodal feature integration during episodic retrieval, significantly greater AnG activity was observed during retrieval of integrated multimodal episodic memories compared with unimodal episodic memories. Multivariate classification analyses revealed that individual multimodal episodic memories could be differentiated in AnG, with classification accuracy tracking the vividness of participants' reported recollections, whereas distinct unimodal memories were represented in sensory association areas only. In contrast to episodic retrieval, AnG was engaged to a statistically equivalent degree during retrieval of unimodal and multimodal semantic memories, suggesting a distinct role for AnG during semantic retrieval. Modality-specific sensory association areas exhibited corresponding activity during both episodic and semantic retrieval, which mirrored the functional specialization of these regions during perception. The results offer new insights into the integrative processes subserved by AnG and its contribution to our subjective experience of remembering.
Significance statement: Using univariate and multivariate fMRI analyses, we provide evidence that functionally distinct subregions of angular gyrus (AnG) contribute to the retrieval of episodic and semantic memories. Our multivariate pattern classifier could distinguish episodic memory representations in AnG according to whether they were multimodal (audio-visual) or unimodal (auditory or visual) in nature, whereas statistically equivalent AnG activity was observed during retrieval of unimodal and multimodal semantic memories. Classification accuracy during episodic retrieval scaled with the trial-by-trial vividness with which participants experienced their recollections. Therefore, the findings offer new insights into the integrative processes subserved by AnG and how its function may contribute to our subjective experience of remembering.
Keywords: fMRI; memory; multivoxel pattern analysis; parietal lobe; recollection.
Copyright © 2016 Bonnici, Richter, et al.
Figures

Comment in
-
The Integration of Distributed Memory Traces.J Neurosci. 2016 Oct 19;36(42):10723-10725. doi: 10.1523/JNEUROSCI.2344-16.2016. J Neurosci. 2016. PMID: 27798126 Free PMC article. No abstract available.
References
-
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–919. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
